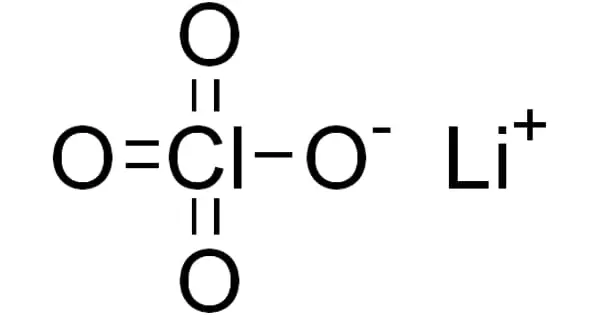
The inorganic compound lithium perchlorate has the formula LiClO4. This white or colorless crystalline salt is notable for its high solubility in a wide range of solvents. It exists as an anhydrous compound as well as a trihydrate.
The high density and availability of oxygen for combustion are significant advantages of lithium perchlorate. The use of lithium perchlorate anhydride complexes in the acylation of activated aromatic compounds is an intriguing method. Lithium perchlorate is frequently used as a promoter to speed up the acylation process and increase yield in metal triflate-catalyzed reactions. The chemical composition of the surface film on lithium metal does not change significantly when lithium perchlorate is used as the electrolyte salt.
Properties
Lithium perchlorate is a colorless, deliquescent crystal of oxysalt. It is a strong oxidizing agent. On a volume basis, it has more available oxygen than liquid oxygen. Lithium perchlorate has a specific gravity of 2.429, making it heavier than water and soluble in water.
- Compound Formula: LiClO4
- Molecular Weight: 106.39
- Appearance: Colorless to white powder or crystals
- Melting Point: 236 °C
- Boiling Point: N/A
- Density: 2.42 g/cm3
- Solubility in H2O: 106.4 g/L (20 °C)
- pH: 6.0-7.5
- Exact Mass: 105.964516
Production
Lithium perchlorate can be manufactured by reaction of sodium perchlorate with lithium chloride. It can be also prepared by electrolysis of lithium chlorate at 200 mA/cm2 at temperatures above 20 °C.

Application in Inorganic chemistry
Lithium perchlorate is used as a source of oxygen in some chemical oxygen generators. It decomposes at about 400 °C, yielding lithium chloride and oxygen:
LiClO4 → LiCl + 2 O2
Over 60% of the mass of the lithium perchlorate is released as oxygen. It has both the highest oxygen to weight and oxygen to volume ratio of all practical perchlorate salts.
Application in Organic chemistry
LiClO4 is extremely soluble in organic solvents, including diethyl ether. Such solutions are used in Diels-Alder reactions, where it is proposed that the Lewis acidic Li+ binds to Lewis basic sites on the dienophile, speeding up the reaction.
Lithium perchlorate is also used as a co-catalyst in the Baylis-Hillman reaction, which involves the reaction of,-unsaturated carbonyls with aldehydes. Under neutral conditions, solid lithium perchlorate is found to be a mild and efficient Lewis acid for promoting the cyanosilylation of carbonyl compounds.
Application in Batteries
In lithium-ion batteries, lithium perchlorate is also used as an electrolyte salt. When superior electrical impedance, conductivity, hygroscopicity, and anodic stability properties are important to the specific application, lithium perchlorate is preferred over alternative salts such as lithium hexafluorophosphate or lithium tetrafluoroborate. These advantageous properties, however, are frequently overshadowed by the electrolyte’s strong oxidizing properties, which make the electrolyte reactive toward its solvent at high temperatures and/or high current loads. Because of these risks, the battery is frequently regarded as unsuitable for industrial applications.
Application in Biochemistry
Concentrated solutions of lithium perchlorate (4.5 mol/L) are used as a chaotropic agent to denature proteins.