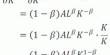Calcium hypochlorite (molecular formula: Ca (ClO)2) appears to be a white granular solid with an odor of chlorine (or tablets compressed from granules). It is a kind of compound that is inorganic. It is the key active ingredient used for water treatment and as a bleaching agent in consumer goods called bleaching powder, chlorine powder, or chlorinated lime. Although it’s far quite strong and non-flammable, it will accelerate the burning of flammable substances.
It is a white solid, but it appears yellow in commercial samples. Because of its slow decomposition in the moist air, it strongly smells of chlorine. Instead, it is highly water-soluble and can be formulated in different amounts. The sodium hypochlorite solutions resulting from this appear as transparent, green to yellow liquids. It has two forms: hydrated (hydrous) and dry (anhydrous).
(Calcium Hypochlorite)
Calcium hypochlorite is a deliquescent white powder, also known as calcium oxychloride, chlorinated lime, and bleach, used as a bleaching agent and as a disinfectant throughout the textile and pulp industries. In the late 18th century, Charles Tennant and Charles Macintosh invented an industrial method for the processing of lime chloride. It turned into patented in 1799 and used closely for the duration of world conflict I for disinfecting the trenches and wounds. Poisonous, aggravating to the pores and skin. Noncombustible, but will boost up the burning of flammable substances.
Calcium hypochlorite is essentially utilized as a dying specialist or disinfectants to clean distribute pools just as purify drinking water. It is additionally utilized in the sanitization of surfaces and gear of the kitchen and restroom. Delayed introduction to fire or warmth may bring about the enthusiastic disintegration of the material and burst of the compartment. Besides, it tends to be utilized as algaecides, herbicide, and clothing cleansers.
Calcium hypochlorite is a popular oxidizing agent and consequently reveals a few uses in natural chemistry. It consists of 60 to sixty-five % to be had chlorine; it could act as a standard oxidizing agent for the cleavage of glycols, keto acids to attain fragmented aldehydes or carboxylic acid. It can likewise be utilized for chloroform handling. Calcium hypochlorite might be utilized in a natural blend to oxidize thiol and sulfide side-effects and accordingly lessen their scent and make them safe to discard.
The use of hypochlorite for the expulsion of dissolvable COD/synthetic oxygen interest/, phenolic and polyphenolic like accumulates, and other natural builds answerable for the olive factory wastewater/olive oil squander water (OOWW)/shading has been tentatively considered. Calcium hypochlorite can be synthetic via the reaction among limes (Ca(OH)2) with chlorine gas to present numerous concentrations of products. The reaction may be performed in tiers to present various compositions, each with different concentrations of calcium hypochlorite, collectively with unconverted lime and calcium chloride. The full conversion is shown:
2 Cl2 + 2 Ca(OH)2 → Ca(ClO)2 + CaCl2 + 2 H2O
Two primary processes, the so-called ‘calcium process’ and the ‘sodium process’, produce calcium hypochlorite. These are exactly the same as the Mg(ClO)2 salt form mentioned above, except that a hypochlorite salt of sodium is used in one. Instead, it is a mixture composed primarily of Ca(ClO)2 calcium hypochlorite, Ca3(ClO)2(OH)4 (also written as Ca(ClO)2 • 2 Ca(OH)2) calcium hypochlorite, and Ca3Cl2(OH)4 (calcium hydroxychloride also written as CaCl2 • 2 Ca(OH)2) dibasic calcium chloride.
As a strong oxidant, calcium hypochlorite may also react with energy in combination with carbon compounds, and combination with finely divided carbon particles bureaucracy an explosive combination. Analysis of the organochloride compounds formed by the reaction between hypochlorite and organic compounds showed that the levels of DDD, DDT, and heptachloride exceeded those recommended by international and European standards for drinking water. Calcium hypochlorite is highly important. It’s also a sturdy oxidizing agent, as it consists of a chlorine atom at the valence I (redox country: Cl+1). It may be found in swimming pool disinfectants, in bleaching marketers, in deodorants, and in fungicides.
Information Sources: