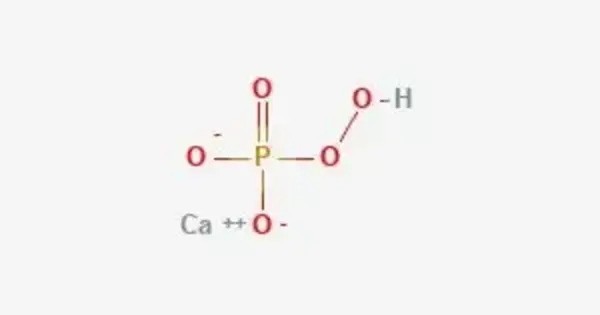
Calcium hydroxyphosphate (calcium phosphate tribasic, tribasic calcium phosphate, hydroxyapatite, HAp) is an inorganic chemical compound that is made up of calcium, hydrogen, oxygen and phosphorus. Its formula is Ca5(OH)(PO4)3. It is a naturally occurring mineral form of calcium apatite. It’s a major component of bone and teeth in humans and other vertebrates.
It also known as hydroxyapatite, is a naturally occurring mineral form of calcium apatite with the formula: Ca10(PO4)6(OH)2. It is a major component of bones and teeth in vertebrates and is also found in some pathological calcifications like kidney stones and arterial plaques. It is found in the body and as the mineral hydroxyapatite.
Properties
- Molar Mass: 1004.6 g/mol
- Solubility: Slightly soluble in water; more soluble in acidic environments
- Crystal System: Hexagonal
- pH Stability: Stable in neutral to basic pH, dissolves in acidic conditions
- Decomposes: At high temperatures to tricalcium phosphate and calcium oxide
- Color: White or pale yellow
- Density: ~3.1 g/cm³
- Hardness: ~5 on Mohs scale
- Structure: Crystalline, hexagonal unit cell
- Thermal Stability: Stable up to ~1,100°C
Natural Occurrence
1. Biological Systems
- Bones and Teeth: Main inorganic component (up to 70% by weight in bones, 96% in enamel).
- Dental Plaque: Can form via mineralization.
- Pathological Calcifications: Appears in kidney stones, gallstones, and arterial plaques.
2. Geological Sources
- Mineral Form: Apatite group, especially hydroxyapatite.
- Found in phosphate rock deposits (often mixed with fluorapatite and chlorapatite).
- Locations: Morocco, Russia, USA, and other phosphate-rich geological areas.
Uses and Applications
- Biomedical: Bone grafts, dental implants, coatings for orthopedic prostheses.
- Dentistry: Toothpaste for remineralization, dental fillings.
- Water Treatment: Removal of heavy metals and fluoride.
- Catalysis: Used in chemical reactions due to ion-exchange properties.