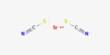
Calcium fumarate is a compound with formula Ca(C2H2(COO)2) or (OOC-CH=CH-COO)Ca. It is a calcium salt of fumaric acid, and has been used to enrich foods to boost calcium absorption. It is primarily used as a nutritional supplement and sometimes as a food additive.
It has E number “E367”.
Properties
- Chemical formula: CaC4H2O4
- Molar mass: 154.134 g mol−1
- Appearance: White to off-white crystalline powder
- Solubility: Sparingly soluble in water
- pH (in aqueous solution): Slightly acidic
- Melting Point: Decomposes upon heating; no distinct melting point
- Stability: Stable under normal conditions; hygroscopic (absorbs moisture from air)
Natural Occurrence and Sources
Not commonly found in nature as a pure mineral, but: Fumarate is a naturally occurring intermediate in the Krebs cycle (citric acid cycle), crucial in cellular respiration.
Calcium fumarate can be synthesized from calcium hydroxide and fumaric acid.
Applications and Uses
- Nutritional Supplement: Source of both calcium and fumarate, often used in fortified foods and dietary supplements.
- Food Additive (as acidity regulator): Registered under E number E367 (not widely used compared to other calcium salts).
- Pharmaceuticals: Sometimes used in formulations to provide calcium and improve tablet properties.
Advantages in Supplements
- Stable and well-tolerated
- Often used where non-animal-derived sources of calcium are required
- May be preferred over calcium carbonate or citrate for some applications due to its specific absorption characteristics or formulation needs