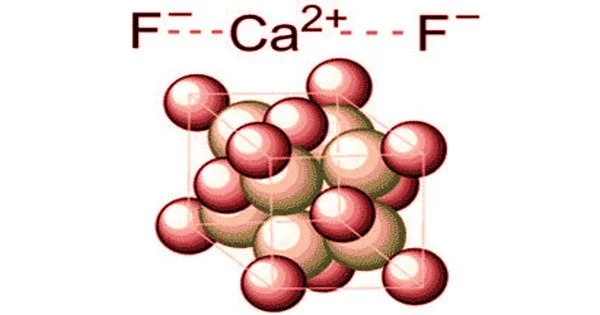
Calcium fluoride is an inorganic compound with the formula CaF2 formed by the elements calcium and fluorine. It is an insoluble white solid. It is found in the mineral fluorite (also known as fluorspar), which is often deeply colored due to impurities. It is a transparent to translucent mineral with colors ranging from intense purple to blue-green to yellow, as well as reddish oranges, pinks, whites, and browns.
Calcium fluoride is used as a flux in the aluminum industry and as a source of fluorine in the production of hydrofluoric acid. It is also employed in cryogenically cooled thermal imaging stages. It can also be used as a gemstone, but its worth is low due to its lack of hardness.
Properties
Calcium fluoride appears as odorless gray powder or granules. It is an insoluble ionic compound of calcium and fluorine. It is used as a flux in the aluminium industry and a source of fluorine for hydrofluoric acid production.
- Chemical formula: CaF2
- Molar mass: 78.075 g·mol−1
- Appearance: White crystalline solid (single crystals are transparent)
- Density: 3.18 g/cm3
- Melting point: 1,418 °C (2,584 °F; 1,691 K)
- Boiling point: 2,533 °C (4,591 °F; 2,806 K)
- Solubility in water: 0.015 g/L (18 °C); 0.016 g/L (20 °C)
- Solubility product (Ksp): 3.9 × 10−11
- Solubility: insoluble in acetone; slightly soluble in acid

Chemical structure
The fluorite structure crystallizes when the compound crystallizes in a cubic motif. Ca2+ centers have eight coordinates and are centered in a cube of eight F− centers. Each F− center is linked to four Ca2+ centers arranged in the shape of a tetrahedron. Although perfectly packed crystalline samples are colorless, due to the presence of F-centers, the mineral is frequently deeply colored. Many ionic compounds with the formula AB2 have the same crystal structure, including CeO2, cubic ZrO2, UO2, ThO2, and PuO2. Anions and cations, such as Be2C, are swapped in the corresponding anti-structure, known as the antifluorite structure.
Preparation
The mineral fluorite is abundant, widespread, and mainly of interest as a precursor to HF. Thus, little motivation exists for the industrial production of CaF2. High purity CaF2 is produced by treating calcium carbonate with hydrofluoric acid:
CaCO3 + 2 HF → CaF2 + CO2 + H2O
Applications
Naturally occurring CaF2 is the principal source of hydrogen fluoride, a commodity chemical used to produce a wide range of materials. Calcium fluoride in the fluorite state is of significant commercial importance as a fluoride source. Hydrogen fluoride is liberated from the mineral by the action of concentrated sulfuric acid:
CaF2 + H2SO4 → CaSO4(solid) + 2 HF
Niche uses
Calcium fluoride is used to make optical components like windows and lenses, which are used in thermal imaging systems, spectroscopy, telescopes, and excimer lasers. It is transparent to a wide range of frequencies, from ultraviolet (UV) to infrared (IR). Because of its low refractive index, it eliminates the need for anti-reflection coatings. Its insolubility in water is also advantageous. Doped calcium fluoride, like natural fluorite, glows under ultraviolet light and is used in thermoluminescent dosimeters. It is formed when fluorine reacts with calcium.
Safety
Fluorides are toxic to humans, but CaF2 is thought to be relatively safe due to its extreme insolubility. The situation is similar to that of BaSO4, where the toxicity of Ba2+ is offset by the very low solubility of its sulfate derivative.