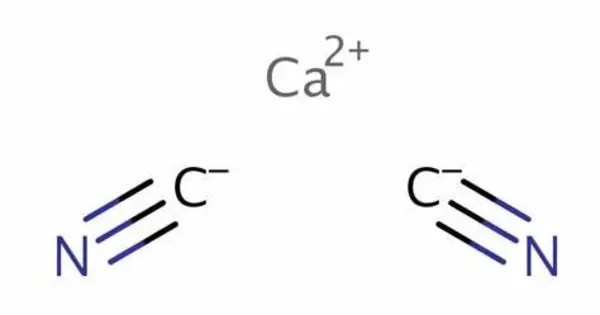
Calcium cyanide is the inorganic compound with the formula Ca(CN)2. It is the calcium salt derived from hydrocyanic acid. It is a white solid, although the pure material is rarely encountered. It’s primarily used in industrial applications, particularly in the production of cyanide compounds, which are important for processes like gold extraction and electroplating.
It slowly hydrolyses in solution or moist air to release hydrogen cyanide and is very toxic. It’s highly toxic and should be handled with extreme caution, as it releases hydrogen cyanide (HCN) gas, which is very poisonous, especially in poorly ventilated areas.
Preparation
Solutions of calcium cyanide can be prepared by treating calcium hydroxide with hydrogen cyanide. Solid calcium cyanide is produced commercially by heating calcium cyanamide with sodium chloride. The reaction is incomplete. The product is only of 50% purity, other components being sodium cyanide, calcium cyanamide, and carbon. Because of the carbon impurity, the solid is black, hence material is often called black cyanide.
Properties
- Chemical formula: Ca(CN)2
- Molar mass: 92.1128 g/mol
- Appearance: white powder
- Odor: hydrogen cyanide
- Density: 1.853 (20 °C)
- Melting point: 640 °C (1,184 °F; 913 K) (decomposes)
- Solubility in water: soluble
- Solubility: soluble in alcohol, weak acids
- Crystal structure: rhombohedric
Chemical Properties
- Reactivity: Calcium cyanide can react with water to produce calcium hydroxide (Ca(OH)₂) and hydrogen cyanide (HCN), a highly toxic gas.
- Toxicity: The cyanide ion (CN⁻) is highly toxic, and exposure to it can interfere with cellular respiration, leading to death in sufficient quantities.
- Stability: It is relatively stable under normal conditions but should be handled with care due to its toxic nature.
Reactivity
At temperatures around 600 °C, calcium cyanide converts to calcium cyanamide:
Ca(CN)2 → CaCN2 + C
It is suspected that this reaction is one step in the conversion of calcium carbide with nitrogen gas. The ratio of calcium cyanide to calcium cyanamide is sensitive to the presence of alkali metal halides, such as sodium chloride.
Calcium cyanide hydrolyzes upon acidification to form hydrogen cyanide:
Ca(CN)2 + 2 H+ → Ca2+ + 2 HCN
Calcium cyanide reacts with ammonium carbonate to give produce ammonium cyanide:
Ca(CN)2 + (NH4)2CO3 → 2 NH4CN + CaCO3
Occurrences
- Natural Occurrences: Calcium cyanide is not commonly found in nature in its pure form. However, cyanide compounds, including cyanides of other metals, can be found in trace amounts in certain minerals or in certain environmental conditions where organic matter undergoes decomposition.
- Industrial Production: It is typically manufactured by reacting calcium carbide (CaC₂) with nitrogen or by reacting calcium oxide (CaO) with carbon in the presence of nitrogen. It is primarily used in the extraction of gold from ores through a process known as cyanidation.
Uses
Calcium cyanide is used almost exclusively in the mining industry. It serves as an inexpensive source of cyanide in many leaching or vat operation to obtain precious metals such as gold and silver from their ores.