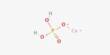
Caesium phosphide refers to any of several inorganic compounds with the formula CsPx. It is an inorganic compound composed of caesium and phosphorus. The most studied member is Cs3P7, which forms yellow crystals of tetragonal structure (P41 group), which turn brown when heated to 300 °C, and colorless when cooled with liquid nitrogen. This compound is usually synthesized by directly combining elemental caesium and phosphorus under controlled conditions, often in an inert atmosphere to prevent unwanted reactions. It is expected to have a high melting point and to be unstable in moist air. Caesium phosphide can act as a semiconductor, similar to related alkali metal phosphides, and may be useful in specialized electronic or photoelectric applications.
It is primarily studied for its chemical properties rather than large-scale industrial use, as its extreme reactivity and the rarity of caesium limit practical applications. However, alkali metal phosphides like Cs₃P are sometimes used as precursors or reagents in research, particularly in the synthesis of other phosphorus-containing materials or for studying alkali metal–phosphorus interactions.
Properties
It typically appears as a dark-colored, crystalline solid and is classified as an ionic phosphide due to its structure of Cs⁺ cations and P³⁻ anions. Like many alkali metal phosphides, caesium phosphide is highly reactive and must be handled with care, especially in the presence of moisture or air. When exposed to water, it undergoes vigorous hydrolysis, releasing phosphine gas (PH₃), which is toxic and flammable.
- Chemical formula: Cs3P7
- Molar mass: 615.53268987 g·mol−1
- Appearance: yellow crystals
Due to its strong reducing properties and ability to release phosphine, caesium phosphide must be stored in tightly sealed, moisture-free containers. Protective equipment is essential when handling it to avoid exposure to its potentially hazardous reaction products.
Occurrences
Caesium phosphide does not occur naturally due to the high reactivity of both caesium and phosphide ions. Instead, it is synthesized in laboratories by directly reacting elemental caesium with phosphorus under controlled conditions, usually in an inert atmosphere to prevent unwanted side reactions. Its instability in water and air limits practical uses, but it is mainly studied in the context of solid-state chemistry, materials science, and potential semiconductor research.