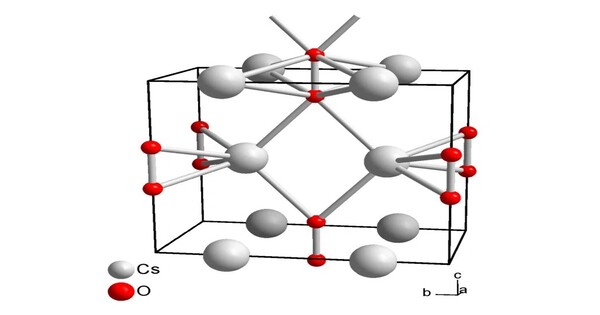
Caesium peroxide or cesium peroxide is an inorganic compound of caesium and oxygen with the chemical formula Cs2O2. It’s highly reactive and unusual peroxide, formed by cesium’s interaction with oxygen. It typically exists as a white or yellowish solid and is part of a larger family of alkali metal peroxides, which are known for their strong oxidative properties.
Properties
It is soluble in water, where it decomposes to form cesium hydroxide (CsOH) and oxygen gas (O₂). It is highly reactive and unstable in moist air or water. It can rapidly break down, releasing oxygen and potentially causing an explosion if not handled properly.
- Chemical formula: Cs2O2
- Molar mass: 297.809 g·mol−1
- Appearance: Yellowish
Formation
2 Cs + O2 → Cs2O2
It can also be formed by the thermal decomposition of caesium superoxide:
2 CsO2 → Cs2O2 + O2
Upon heating until 650 °C, the compound will decompose to caesium monoxide and atomic oxygen:
Cs2O2 → Cs2O + [O]
Caesium peroxide shows a Raman vibration at 743 cm−1, due to the presence of the peroxide ions. The compound is often used as a coating for photocathodes, due to its low work function.
Reactivity
Caesium peroxide reacts vigorously with water, producing cesium hydroxide (CsOH) and releasing oxygen gas. This reaction is exothermic and can be hazardous. It can also react with acids to form cesium salts and hydrogen peroxide (H₂O₂).
Caesium peroxide is a strong oxidizer, particularly when it decomposes or interacts with other substances.
Occurrences
Caesium peroxide does not occur naturally in large quantities. However, cesium (the metal from which caesium peroxide is derived) can be found in trace amounts in minerals such as pollucite, which is the primary natural source of cesium. The formation of caesium peroxide may occur synthetically in laboratories or in controlled industrial environments rather than in nature.
Synthetic Production
Caesium peroxide is typically synthesized by reacting caesium metal with oxygen under controlled conditions, or by reacting cesium hydroxide (CsOH) with oxygen-rich compounds at high temperatures.
Applications
Due to its strong oxidizing properties, caesium peroxide may have potential uses in research and in situations requiring the generation of oxygen or the handling of reactive oxygen species. However, it is not as commonly used as other peroxides due to its instability and the handling difficulties posed by its reactivity.