Caesium ozonide is an oxygen-rich chemical compound of caesium, with the chemical formula CsO3. It consists of caesium cations Cs+ and ozonide anions O−3. It can be formed by reacting ozone with caesium superoxide:
CsO2 + O3 → CsO3 + O2
The compound reacts strongly with any water in the air forming caesium hydroxide.
4 CsO3 + 2 H2O → 4 CsOH + 5 O2
Ozonides are typically unstable and reactive because of the presence of the O₃⁻ ion, which is a high-energy species. Caesium ozonide, like other alkali metal ozonides, can be synthesized by reacting caesium metal with ozone gas (O₃) under controlled conditions, often at low temperatures.
Because of its high reactivity, caesium ozonide is not a compound that is widely encountered in everyday situations and requires careful handling.
Properties
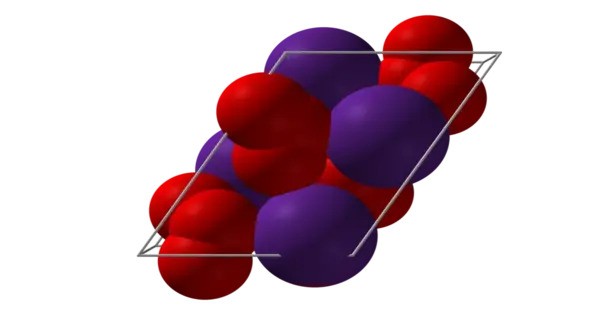
Caesium ozonide typically appears as a blue or blue-green solid, though it can be somewhat unstable. It is highly reactive and unstable, especially when exposed to light or heat. It can decompose to form caesium oxide (Cs₂O) and molecular oxygen (O₂). This reaction is exothermic and can release a significant amount of energy. It is soluble in polar solvents like water, but it decomposes quickly in the presence of moisture.
- Chemical formula: CsO3
- Molar mass: 180.902 g·mol−1
- Appearance: Dark cherry red powder
- Density: 3.19 g/cm3
- Melting point: 85 °C (185 °F; 358 K) (decomposes)
If heated to between 70 and 100 °C, caesium ozonide will quickly decompose to caesium superoxide (CsO2). In fact, the compound is metastable to decomposition into caesium superoxide, slowly decomposing at room temperature, but can remain intact for months if stored at −20 °C.
Structure
The compound contains the ozonide ion (O₃⁻), which is an unusual form of oxygen where three oxygen atoms are bonded in a bent structure. The ozonide anion is generally unstable, especially in compounds with highly reactive metals like caesium.
Occurrences
- Synthesis: Caesium ozonide is not typically found in nature but can be synthesized in laboratories. It is generally produced by reacting caesium metal with ozone (O₃) under controlled conditions.
- Related compounds: Ozonides of other alkali metals, such as lithium ozonide (LiO₃), sodium ozonide (NaO₃), and potassium ozonide (KO₃), share similar properties but vary in stability and reactivity depending on the metal involved.