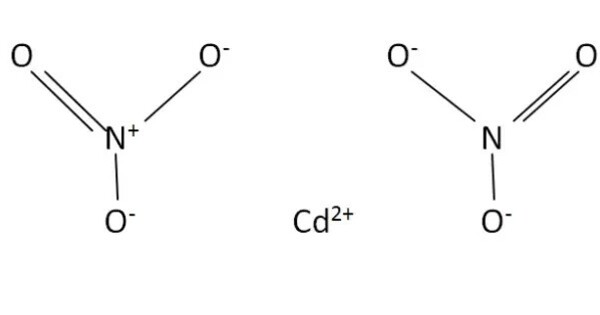
Cadmium nitride is a nitride of cadmium with the chemical formula Cd3N2. It is an inorganic compound composed of cadmium and nitrogen. It typically has a complex crystalline structure, often existing as a yellow-brown solid. It is generally insoluble in water but may react with acids to release nitrogen gas. It can decompose at high temperatures, releasing nitrogen gas.
Cadmium compounds are toxic, and cadmium nitride poses health risks. Care should be taken in handling it to avoid exposure.
Properties
Cadmium nitride is a black solid that decomposes on contact with water and air. It will explode when reacting with dilute acids or alkalis. It is a crystal of inverse manganese(III) oxide structure with lattice constant a = 1079 pm.
- Chemical formula: Cd3N2
- Molar mass: 365.256 g·mol−1
- Appearance: black solid
- Density: 7.67 g·cm−3
- Solubility: Poorly soluble in water
- Thermal Stability: Can decompose upon heating
Preparation
Cadmium nitride can be produced by thermal decomposition of cadmium amide at 180 °C:
3 Cd(NH2)2 → Cd3N2 + 4 NH3
It can also be produced by thermal decomposition of cadmium azide at 210 °C.
Synthesis
Cadmium nitride can be synthesized through various methods, including direct reaction of cadmium and nitrogen at elevated temperatures.
Natural Occurrences
Cadmium nitride is not commonly found in nature as a distinct mineral, but cadmium itself occurs in various minerals like greenockite.
Applications
While not as widely used as other cadmium compounds, cadmium nitride has potential applications in semiconductors, ceramics, and as a precursor for other cadmium-based materials.
- Semiconductors: Cd3N2 has potential uses in the development of semiconductor materials.
- Photovoltaics: Research is ongoing into its applications in solar energy conversion.
- Catalysts: It may also be explored for its catalytic properties.
Safety Considerations
Due to the toxic nature of cadmium and its compounds, handling cadmium nitride requires strict safety protocols, including personal protective equipment and proper waste disposal methods.