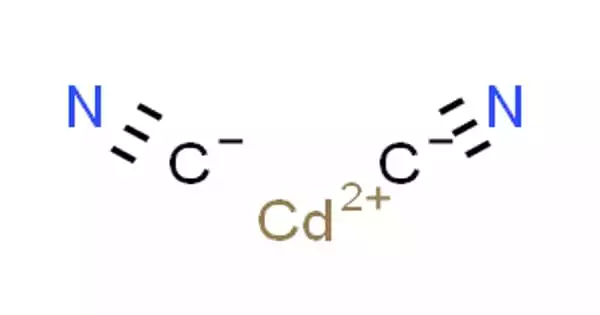
Cadmium cyanide, also known as Cd(CN)2, is an inorganic compound. It is a flexible cubic coordination polymer that has previously been shown to contract when heated. It is a crystalline white compound used in electroplating. It, along with other cadmium and cyanide compounds, is extremely toxic.
Cadmium cyanide is a cadmium and cyanide chemical compound that is formed by reacting a concentrated aqueous solution of cadmium chloride or cadmium nitrate with potassium cyanide or sodium cyanide. It is used as an electrolyte for the electrodeposition of thin metallic cadmium coatings on metal in order to protect it from corrosion. Cadmium is a transition metal and chemical element with the atomic number 48 and the symbol Cd. It is naturally found in the earth’s crust, but only rarely on its own.
Preparation and structure
Cadmium cyanide is prepared commercially by treating cadmium hydroxide with hydrogen cyanide:
Cd(OH)2 + 2 HCN → Cd(CN)2 + 2 H2O
It can also be generated from tetracyanocadmate:
[Cd(CN)4]2- + CdCl2 → 2 Cd(CN)2 + 2 Cl–
Cadmium cyanide can be made by combining a concentrated aqueous solution of cadmium chloride or cadmium nitrate with potassium cyanide or sodium cyanide. The resulting white precipitate is filtered, washed, and dried.
CdCl2 + 2KCN → Cd(CN)2 + 2KCl.
Cadmium cyanide and zinc cyanide have structures that are similar. Each metal, as a result, has a tetrahedral coordination sphere. Cyanide ligands connect two metal centers. Interpenetration exists between two of the resulting diamondoid structures. The structure is similar to that of cristobalite, a SiO2 polymorph. The structural similarity of cadmium dicyanide and cristobalite was instrumental in the development of mineralomimetic chemistry, which is defined as “the formation of mineral-like structures using materials that never give stable minerals.”
Reactions and uses
It is used as an electrolyte for the electrodeposition of thin metallic cadmium coatings on metal in order to protect it from corrosion.
Cadmium cyanide, like zinc cyanide, is water-soluble, which is unusual for transition metal cyanides. With the addition of cyanide, the solubility increases, with the reaction taking place via “[Cd(CN)3]–” and [Cd(CN)4]2-. Its solutions evolve hydrogen cyanide when exposed to acids. Clathrates form when it crystallizes in the presence of certain small molecules.