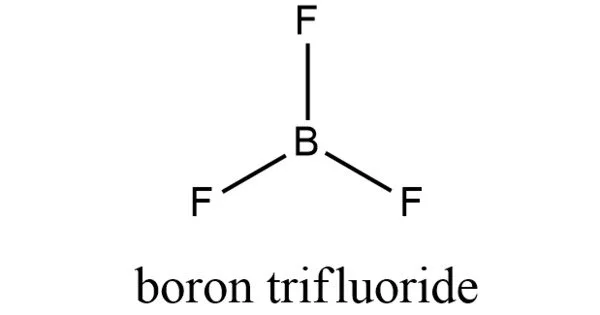
Boron trifluoride, (BF3), is an inorganic compound. It has a strong odor and is colorless. In moist air, this pungent, colorless toxic gas emits white fumes. It is water-soluble and is slowly hydrolyzed by cold water to produce hydrofluoric acid, a corrosive material. It functions as a Lewis acid and a versatile building block for other boron compounds. It is toxic when inhaled. Its vapors weigh more than air.
Boron trifluoride is a colorless, nonflammable gas with an acrid, suffocating odor. In moist air, it emits thick acidic fumes. Dry boron trifluoride is used in the production of mild steel, copper, copper-zinc, copper-silicon, and nickel alloys.
Properties
Boron trifluoride is an odorless, colorless toxic gas. It has melting and boiling points of -127 and -100 degrees Celsius, respectively. It has a density of 0.90 g mL-1. It dissolves in water. Sulfuric acid and organic solvents such as benzene, carbon tetrachloride, sulfur dioxide, and chloroform are also soluble in it.
- Chemical formula: BF3
- Molar mass: 67.82 g/mol (anhydrous); 103.837 g/mol (dihydrate)
- Appearance: colorless gas (anhydrous); colorless liquid (dihydrate)
- Density: 0.00276 g/cm3 (anhydrous gas); 1.64 g/cm3 (dihydrate)
- Melting point: −126.8 °C (−196.2 °F; 146.3 K)
- Boiling point: −100.3 °C (−148.5 °F; 172.8 K)
- Solubility in water: exothermic decomposition (anhydrous); very soluble (dihydrate)
- Solubility: soluble in benzene, chloroform and methylene chloride
Boron trifluoride is extremely corrosive. Metals suitable for boron trifluoride handling equipment include stainless steel, monel, and hastelloy. It corrodes steel, including stainless steel, in the presence of moisture. It combines with polyamides. Polytetrafluoroethylene, polychlorotrifluoroethylene, polyvinylidene fluoride, and polypropylene all exhibit adequate resistance. Because boron trifluoride reacts with hydrocarbon-based greases, the grease used in the equipment should be fluorocarbon-based.
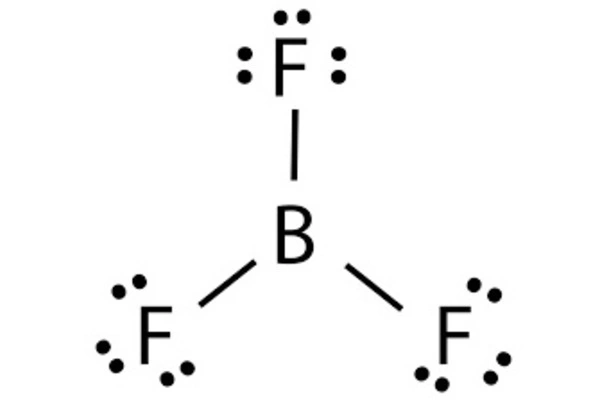
Structure and bonding
The geometry of a molecule of BF3 is trigonal planar. Its D3h symmetry conforms with the prediction of VSEPR theory. The molecule has no dipole moment by virtue of its high symmetry.
In the boron trihalides, BX3, the length of the B–X bonds (1.30 Å) is shorter than would be expected for single bonds, and this shortness may indicate stronger B–X π-bonding in the fluoride. A facile explanation invokes the symmetry-allowed overlap of a p orbital on the boron atom with the in-phase combination of the three similarly oriented p orbitals on fluorine atoms. Others point to the ionic nature of the bonds in BF3.
Reactions
Unlike the aluminium and gallium trihalides, the boron trihalides are all monomeric. They undergo rapid halide exchange reactions:
BF3 + BCl3 → BF2Cl + BCl2F
Because of the facility of this exchange process, the mixed halides cannot be obtained in pure form.
Boron trifluoride is a versatile Lewis acid that forms adducts with such Lewis bases as fluoride and ethers:
CsF + BF3 → CsBF4
O(C2H5)2 + BF3 → BF3·O(C2H5)2
Tetrafluoroborate salts are commonly employed as non-coordinating anions. The adduct with diethyl ether, boron trifluoride diethyl etherate, or just boron trifluoride etherate, (BF3·O(Et)2) is a conveniently handled liquid and consequently is widely encountered as a laboratory source of BF3. Another common adduct is the adduct with dimethyl sulfide (BF3·S(Me)2), which can be handled as a neat liquid.
Uses
Boron trifluoride is used to make HBF4, an organic chemistry catalyst. It’s also employed as a fluorinating agent. It is also used as a catalyst in alkylation, esterification, and condensation reactions. Some boron compounds, such as diborane, are prepared using BF3.
Health effects/safety hazards
Boron trifluoride is extremely toxic when inhaled. It causes severe eye and skin damage. When BF3 reacts with water, it produces highly corrosive hydrofluoric acid. When heated, it emits white toxic fluoride fumes. When it comes into contact with water, it decomposes. Plastic and rubber may be attacked by BF3.