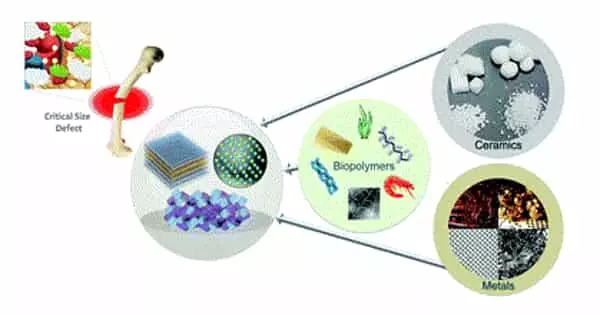
Bone is a tissue that can heal and regenerate on its own. In orthopedics and traumatology, a bone defect can form on occasion, and in this case, the bone fails to heal and requires bone reconstruction. Osteoproduction, osteoinduction, osteoconduction, mechanical stimulation, and vascularisation are all required for successful bone reconstruction. In addition, drugs that affect bone metabolism can play an important role in bone ingrowth.
Bone injuries, vehicle accidents, and battlefield injuries can all result in bone injuries to the face and skull, known as craniomaxillofacial defects. Repairing such flaws is difficult because various types of cells must interact with one another. In a new study, researchers are looking into the different types of materials used in reconstruction to see which one is the most effective.
Every year, over 2 million bone graft surgeries are performed around the world. Because CMF bone defects are typically irregular in shape, they are frequently repaired with regenerative biomaterials. The Harley Lab creates collagen scaffold biomaterials that contain bone-like components such as calcium and phosphate ions, as well as sugar compounds known as glycosaminoglycans (GAGs).
“Our lab is interested in developing degradable biomaterials, also known as scaffolds, for bone and tissue repair,” said Vasiliki Kolliopoulos, a graduate student in the Harley lab. “Many different types of cells in the bone environment aid in healing, including stem cells that form bone and monocytes that aid in the immune response. The purpose of this study was to see how the scaffold material affects the combined behavior of these different cells.”
In a new study, researchers are investigating the types of material used in bone reconstruction to see which one works best.
The researchers modified a collagen biomaterial to include one of three GAGs found in the bone tissue microenvironment: chondroitin-4-sulfate, chondroitin-6-sulfate, and heparin. They then looked into how these GAGs affect processes important for bone regeneration, such as stem cell activity, immune cell activation, and endothelial cell activity, which is required for blood vessel formation.
To accomplish this, stem cells were added to the scaffolds and the surrounding solution, or media, was collected for up to 21 days. Stem cells are potent molecule factories that can influence other cells in the wound environment. The conditioned media was then added to cultures of endothelial cells, which are found in blood vessels. “Bone regeneration requires blood vessel growth, and few people have looked at how to scaffold materials affect endothelial cells and how it could improve bone repair,” said Marley Dewey, a former graduate student in the Harley lab.
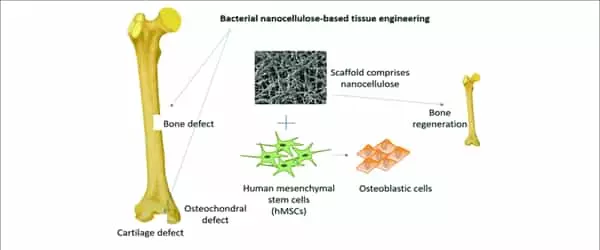
Understanding the composition and structure of native bone tissue, as well as the appropriate selection of biomimetic natural or tunable synthetic materials (biomaterials) such as polymers, bioceramics, metals, and composites, are required for successful materials design for bone-tissue engineering. Scalable fabrication technologies, such as three-dimensional printing and electric-field-assisted techniques, that allow control over construct architecture at multiple length scales, can then be used to process these biomaterials into suitable forms for bone-tissue engineering.
For 6-12 hours, the researchers monitored the growth of endothelial cells. “Despite the fact that heparin is known to directly influence blood vessel formation, we were surprised to see that the media generated by stem cells in chondroitin-6-sulfate scaffolds led to the greatest amount of blood vessel development compared to the other two scaffolds,” Kolliopoulos said.
The conditioned media was also investigated to identify the molecules known as soluble factors that aid in blood vessel and bone development. Finally, the researchers added the conditioned media to monocytes and monitored their growth for 21 days to determine the types of immune cells they developed. They discovered that the types and numbers of soluble factors differed depending on the scaffold type, with chondroitin-6-sulfate media producing the greatest number of immune cells that aid in the inflammatory response.
The researchers intend to look into the immune cells’ responses further. “When your body sounds the alarm that something is wrong, stem cells can send a signal to monocytes. As a result, we want to see if stem cells grown in scaffolds in an inflammatory environment secrete a different cocktail of soluble factors “According to Kolliopoulos.
“These findings indicate that soluble factors play an important role in these multicellular systems,” said Kolliopoulos. “We demonstrated that cell responses differ depending on the material used, and it is critical to understand these interactions before moving on to more complicated experiments.”
It is unclear which aspect of the scaffold material is causing the differences in growth factors and cell growth, which is a problem that the Harley lab intends to address next. “Once we’ve determined how the scaffolds influence the cells, we’d like to combine different cell types to see what happens,” Kolliopoulos explained. “We’re working to create biomaterials that surgeons can use to repair bone defects. It is critical to comprehend what these materials do to various cell types.”