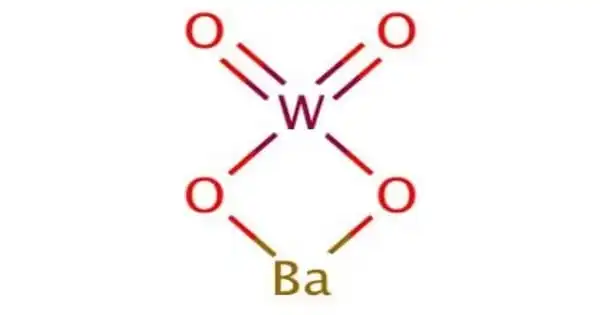
Barium tungstate is an inorganic chemical compound of barium and the tungstate anion. It is a toxic, white powder used as a pigment and in x-ray photography. It is also known as barium white; barium wolframate; tungstate white; wolfram white.
Properties
- Molecular Weight: 385.16
- Appearance: solid
- Melting Point: 1502 0C
- Boiling Point: N/A
- Density: 5.04 g/cm3
- Solubility in H2O: N/A
- Color: White powder

Synthesis
Barium tungstate can be obtained from the precipitation reaction between barium nitrate and ammonium paratungstate or sodium tungstate.
Ba(NO3)2 + Na2WO4 → BaWO4↓ + 2 NaNO3
It is a white solid, which at normal conditions forms tetragonal crystals similar to scheelite, CaWO4. Under pressures above 7 GPa, the compound undergoes transformation to a monoclinic structure similar to fergusonite, YNbO4.
Barium tungstate (BaWO4) crystallites were successfully synthesized at room temperature via an easily-manipulated electrochemical method, using tungsten plates as electrodes and Ba(OH)2 solution as electrolyte. Effects of electric current value and reaction time on the crystallization and development of BaWO4 crystallites were initially studied and characterized by X-ray diffraction (XRD), scanning electron microscopy, and photoluminescent (PL) spectra technique, respectively.
Uses
Barium tungstate can be used as a frequency shifter in laser technology. It has uses in X-ray photography and as a pigment. It is regularly used in pigment and in X-ray photography for manufacturing of intensifying and phosphorescent screens.