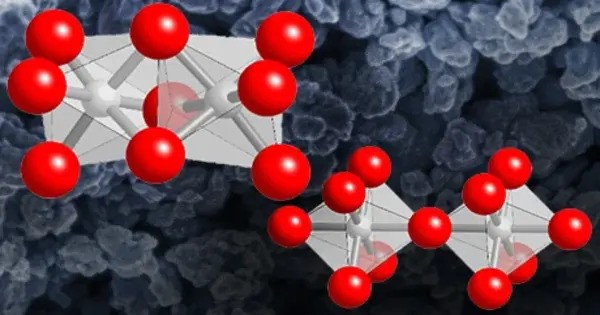
Barium ruthenate is an inorganic compound, with the chemical formula BaRuO3. It belongs to the family of ruthenates, which are transition-metal oxides well-known for their unusual electronic, magnetic, and catalytic properties. It can be obtained from the stoichiometric reaction of barium oxide and ruthenium(IV) oxide at temperatures below 1200 °C, or from the thermal decomposition of Ba[Ru(NO)(NO2)4(OH)]·xH2O.
It reacts with ruthenium and ruthenium(IV) oxide at 1250 °C to obtain black needle-like crystal BaRu6O12. Hydrogen or zirconium can reduce it when heated to produce metal ruthenium. It is a synthetic perovskite oxide with multiple structural polymorphs, displaying unusual electrical and magnetic properties. It does not occur naturally but is prepared in labs for fundamental research and potential catalytic and electronic applications.
Properties
- Chemical formula: BaO3Ru
- Molar mass: 286.39 g·mol−1
- Appearance: black solid
- Density: ~6–7 g/cm³ (varies with polymorph).
- Thermal Stability: High melting point; stable at elevated temperatures in air.
- Insoluble in water; stable in most common solvents.
Electronic and Magnetic Properties
- Conductivity: Can exhibit metallic, semiconducting, or insulating behavior depending on stoichiometry and structure.
- Magnetic Behavior: Some forms of barium ruthenate show paramagnetism or weak ferromagnetism due to Ru 4d electron interactions.
- Exhibits interesting correlated electron phenomena (similar to SrRuO₃ and CaRuO₃).
Synthesis
- Usually prepared by solid-state reaction of barium carbonate (BaCO₃) and ruthenium dioxide (RuO₂) at high temperatures (900–1200 °C) in an oxygen atmosphere.
- Can also be made via sol–gel or hydrothermal routes for fine powders.
Occurrences
Barium ruthenate does not occur naturally; it is a synthetic compound made in laboratories. Its family members (ruthenates) are mainly studied for physics and materials science research rather than being naturally found minerals.
Applications
- Catalysis – Ruthenium oxides are known for catalytic activity (e.g., in oxidation reactions, fuel cells, and water splitting).
- Electronic Materials – Due to perovskite structure, potential use in oxide electronics, thin films, and resistors.
- Magnetism & Superconductivity Research – Related ruthenates show exotic ground states, making BaRuO₃ useful for condensed matter studies.
- Energy Devices – Possible applications in solid oxide fuel cells (SOFCs) and electrochemical devices.