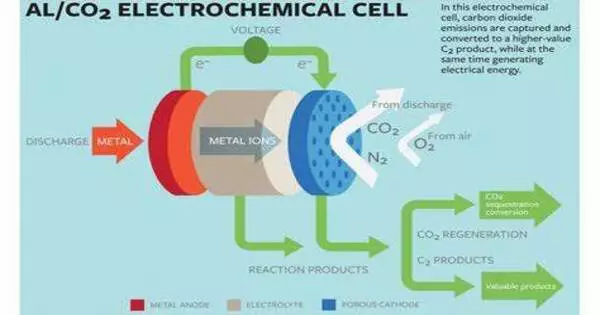
Researchers devised an effective method for converting carbon dioxide into formate, a nonflammable liquid or solid chemical that, like hydrogen or methanol, may be used to run a fuel cell and create energy.
The globe is looking for solutions to capture carbon dioxide from the air or power plant exhaust and convert it into something useful. One of the most intriguing concepts is to turn it into a stable fuel that may be used in place of fossil fuels in certain applications. However, most such conversion processes have had issues with low carbon efficiency or producing fuels that are difficult to handle, poisonous, or combustible.
Researchers at MIT and Harvard University have devised an effective process for converting carbon dioxide into formate, a liquid or solid chemical that can be used to run a fuel cell and create electricity in the same way as hydrogen or methanol can. Potassium or sodium formate, which is already produced on a large scale and is routinely used as a de-icer for roads and sidewalks, is nontoxic, nonflammable, easy to store and transport, and can remain stable in standard steel tanks for months, if not years, after production.
The new method, created by MIT doctoral students Zhen Zhang, Zhichu Ren, and Alexander H. Quinn, Harvard University doctorate student Dawei Xi, and MIT Professor Ju Li, was published in the journal Cell Press Physical Sciences this week. The entire process was performed on a small laboratory scale, including gas capture and electrochemical conversion to a solid formate powder, which is subsequently utilized in a fuel cell to generate energy. However, the researchers anticipate that it will be scalable, allowing it to offer emissions-free heat and power to individual houses as well as industrial or grid-scale applications.
The highly concentrated liquid potassium or sodium formate solution can then be dried, for example, by sun evaporation, to generate a solid powder that is highly stable and can be stored in standard steel tanks for years or even decades.
Professor Ju Li
Other methods of turning carbon dioxide into fuel, according to Li, often entail a two-stage process: The gas is first chemically trapped and converted to a solid form as calcium carbonate, then heated to drive out the carbon dioxide and convert it to a fuel source such as carbon monoxide. According to Li, the second stage is inefficient, turning less than 20% of the gaseous carbon dioxide into the desired product.
By initially turning carbon dioxide into an intermediate state, liquid metal bicarbonate, the novel process achieves a conversion rate of well over 90% and eliminates the need for an inefficient heating phase. In an electrolyzer that employs low-carbon electricity, such as nuclear, wind, or solar power, the liquid is electrochemically transformed into liquid potassium or sodium formate. According to Li, the highly concentrated liquid potassium or sodium formate solution can then be dried, for example, by sun evaporation, to generate a solid powder that is highly stable and can be stored in standard steel tanks for years or even decades.
Several steps of optimization developed by the team made all the difference in changing an inefficient chemical conversion process into a practical solution, says Li, who holds joint appointments in the departments of Nuclear Science and Engineering and Materials Science and Engineering.
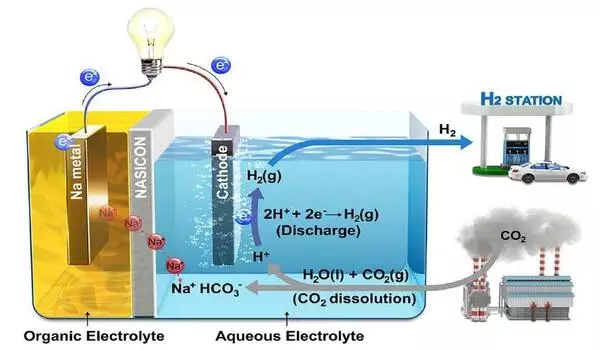
The carbon capture and conversion process begins with an alkaline solution-based capture that concentrates carbon dioxide into the form of a liquid metal-bicarbonate solution, either from concentrated streams such as power plant emissions or from very low-concentration sources such as open air. The bicarbonate is then electrochemically transformed into solid formate crystals with a carbon efficiency of more than 96 percent using a cation-exchange membrane electrolyzer, as proven by the team’s lab-scale studies.
These crystals have an infinite shelf life and are so stable that they can be stored for years, if not decades, with little or no loss. According to Li, even the finest available practical hydrogen storage containers enable the gas to seep out at a rate of around 1% per day, ruling out any uses that would necessitate year-long storage. Methanol, another widely investigated alternative for turning carbon dioxide into a fuel suitable for use in fuel cells, is a poisonous chemical that cannot be easily adapted for use in settings where leakage could represent a health risk. Formate, on the other hand, is widely used and regarded as safe by national safety regulations.
Several improvements account for the greatly improved efficiency of this process. First, a careful design of the membrane materials and their configuration overcomes a problem that previous attempts at such a system have encountered, where a buildup of certain chemical byproducts changes the pH, causing the system to steadily lose efficiency over time. “Traditionally, it is difficult to achieve long-term, stable, continuous conversion of the feedstocks,” Zhang says. “The key to our system is to achieve a pH balance for steady-state conversion.”
To do this, the researchers used thermodynamic modeling to create a new method that is chemically balanced and maintains a constant pH with no acidity shift over time. As a result, it can continue to operate efficiently for extended periods of time. During their tests, the system worked for more than 200 hours with no noticeable decline in output. The entire procedure can be carried out at room temperature and at relatively low pressures (approximately five times atmospheric pressure).
Another issue was that undesirable side reactions produced additional chemical compounds that were not useful, but the researchers solved this by adding an extra “buffer” layer of bicarbonate-enriched fiberglass wool that prevented these reactions.
The team also built a fuel cell specifically optimized for the use of this formate fuel to produce electricity. The stored formate particles are simply dissolved in water and pumped into the fuel cell as needed. Although the solid fuel is much heavier than pure hydrogen, when the weight and volume of the high-pressure gas tanks needed to store hydrogen is considered, the end result is an electricity output near parity for a given storage volume, Li says.
According to the researchers, the formate fuel has the potential to be adapted for everything from home-sized units to large-scale industrial purposes or grid-scale storage systems. In the beginning, a refrigerator-sized electrolyzer machine could capture and convert carbon dioxide into formate, which could then be stored in an underground or rooftop tank. The powdered substance would then be combined with water and fed into a fuel cell to create electricity and heat as needed. “This is for community or household demonstrations,” Zhang said, “but we believe that also in the future it may be good for factories or the grid.”