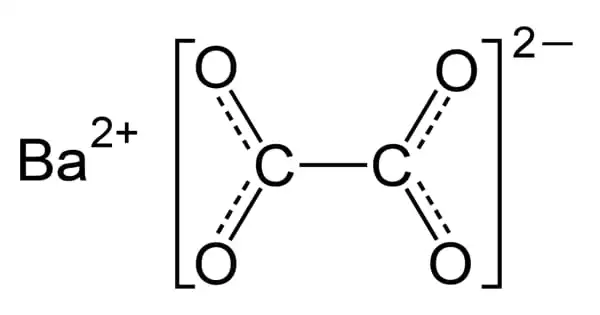
Barium oxalate (BaC2O4) is a white, odorless powder that is sometimes used as a green pyrotechnic colorant in specialist pyrotechnic compositions, including magnesium metal powder. Without the addition of additional chlorine donors, the flame color is rich and brilliant. The burn rate of such compositions is met without the usage of regularly used oxidizers such as nitrates, chlorates, and perchlorates.
Despite being rather stable, barium oxalate can be reactive with strong acids. The chemical, a moderate skin irritant, is poisonous when ingested, producing nausea, vomiting, kidney failure, and gastrointestinal tract harm.
Properties
Barium oxalate is a white, odorless powder that is occasionally employed as a green pyrotechnic colorant in specialized pyrotechnic compositions utilizing magnesium. It is a reducing agent rather than an oxidizing agent, which distinguishes it from most pyrotechnic colorants.
It is a reducing agent rather than an oxidizing agent, which distinguishes it from most pyrotechnic colorants. It is exceedingly insoluble in water and, when heated, transforms to the oxide form.
- Molecular Weight: 225.346
- Appearance: White
- Melting Point: 400 °C
- Boiling Point: N/A
- Density: 2.66 g/cm3
- Solubility in H2O: N/A

Preparation
The raw materials that are required to prepare barium oxalate are oxalic acid and barium hydroxide (or its octahydrate).
It can also be prepared by using an oxalic acid solution and a barium chloride solution, with the reaction as follows:
BaCl2 + H2C2O4 → BaC2O4↓ + 2 HCl
Oxalic acid, Barium hydroxide octahydrate, and Barium hydroxide are the raw materials needed to make Barium oxalate. It differs from the majority of pyrotechnic colorants in that it is a reducing agent rather than an oxidizing agent. It is exceedingly insoluble in water and, when heated, transforms to the oxide form.
Application
By heating in air, barium oxalate crystallizes as the octahydrate, which can then be transformed to the monohydrate. The anhydrous hydroxide has only minor industrial value; the monohydrate and octahydrate are significantly more often employed.
Barium oxalate, particularly the monohydrate, is used to make organic barium compounds such as oil additives and plastic stabilizers. Furthermore, Barium oxalate is employed for dehydration and deacidification, particularly in the removal of sulfuric acid from fats, oils, waxes, and glycerol.