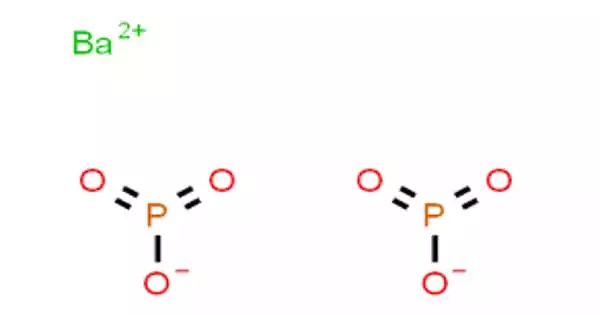
The inorganic compound barium metaphosphate has the chemical formula Ba(PO3)2. It is a colorless solid that is insoluble in water but soluble in acidic solutions via “slow dissolution.” This substance is made of Ba2+ cations linked to a polyphosphate ((PO3-)n) anion, according to X-ray crystallography. There are several hydrated forms that are truly cyclic metaphosphates, such as Ba2(P4O12)•3.5H2O and Ba3(P3O9)2•6H2O. Barium metaphosphate is a flame retardant and phosphor that is used in glasses, porcelains, and enamels.
Properties
- Molecular Weight: 295.27
- Appearance: Powder
- Melting Point: 1560 °C (lit.)
- Boiling Point: N/A
- Density: 3.63 g/cm3
- Solubility in H2O: N/A
- Form: Powder

Preparation
Barium metaphosphate can be prepared by the reaction of barium carbonate with metaphosphoric acid:-
BaCO + 2HPO3 → Ba(PO3)2 + CO2 + H2O
or alternatively by the aqueous reaction of barium chloride and sodium metaphosphate:- BaCl2(aq) + 2NaPO3(aq) → Ba(PO3)2 + 2NaCl
The optimal temperature to utilize is around 700°C. It has the CAS number 13762-83-9 and a molecular weight of 295.2709 g/mol. When diammonium phosphate is used instead of solid metaphosphoric acid, the result is not pure. Colored glasses are formed as a result of the reduction of P-atoms in the phosphate anion by hydrogen released from NH3 to form colloidal dispersions of P4, which produce varied hues in the glass. The finished product is b-Ba(PO3)2. The following aqueous processes can also be used to produce this compound:
Or,
Ba(OH)2 (solid) + 2HPO3 (aq) → Ba(PO3)2 (solid) + 2H2O
HPO3 dissolution and reaction are slow and should be performed in a hot solution. The crystals are formed through evaporation at a low temperature (<15°C). Depending on the precipitation conditions, the result contains different hydrates. Anhydrous barium metaphosphate is insoluble in water white porous powder or granular substance.
Applications
When sodium and barium polyphosphate are combined, they generate a low-melting glass with a high coefficient of thermal expansion. The melting point of glass rises as barium content rises. This glass is used to manufacture seals with low melting metals such as aluminum (melting point 650 °C).
Because barium metaphosphate coupled with sodium has a high coefficient of thermal expansion, it is commonly utilized in the production of low-melt glass as well as other specialties / optical glasses such as fluorophosphate optical glass. Ordinary borosilicate glasses weaken when heated over the melting point of aluminum. A mixture of diammonium phosphate, sodium carbonate, and barium carbonate is heated to create this mixture.