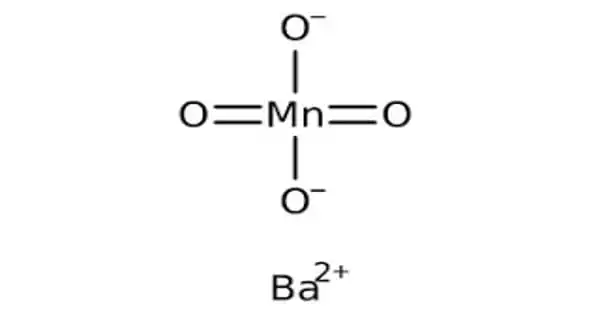
Barium manganate has the formula BaMnO4 and is an inorganic compound. It is a type of manganate in which the manganese is oxidized to +6. It is made by combining manganese dioxide with barium carbonate or nitrate, or by precipitating potassium manganate with barium chloride.
The green pigment produced by barium manganate was used to create manganese blue. Barium manganate can be used to efficiently and selectively oxidize a wide range of functional groups, including alcohols to carbonyls, alcohols to aldehydes, diols to lactones, thiols to disulfides, aromatic amines to azo-compounds, hydroquinone to p-benzoquinone, benzylamine to benzaldehyde, and others.
Properties
The manganate(VI) ion is a d1 ion and is tetrahedral with bond angles of approximately 109.5°. The Mn-O bond lengths in BaMnO4 and K2MnO4 are identical at 1.66 Å. In comparison, the Mn-O bond length in MnO42- is longer than in MnO4– of 1.56 Å and shorter than the Mn-O bond found in MnO2, 1.89 Å. Barium manganate is isomorphous with BaCrO4 and BaSO4. Barium manganate can appear as dark blue or green to black crystals. Barium manganate is indefinitely stable, active, and can be stored for months in dry conditions.
- Molecular Weight: 256.26
- Appearance: Blue to black powder or chunks
- Melting Point: N/A
- Boiling Point: N/A
- Density: 4.85 g/cm3
- Form: Fine Crystalline Powder
- Water Solubility: Slightly soluble in water
- Sensitive: Moisture Sensitive

Preparation
Barium manganate can be prepared from potassium manganate and barium chloride by salt metathesis to give insoluble barium manganate:
BaCl2 + K2MnO4 → 2 KCl + BaMnO4↓
Application
In organic chemistry, it is used as an oxidant. It is a manganate compound, which means that the manganese is in an oxidation state of +6. Manganate must not be confused with permanganate, which also contains manganese (VII). Barium manganate is a strong oxidant that is widely used in organic synthesis and can be used in a wide range of oxidation reactions.
Uses in organic synthesis
Barium manganate efficiently and selectively oxidizes a variety of functional groups, including alcohols to carbonyls, diols to lactones, thiols to disulfides, aromatic amines to azo compounds, hydroquinone to p-benzoquinone, benzylamine to benzaldehyde, and others. Saturated hydrocarbons, alkenes, unsaturated ketones, and tertiary amines are not oxidized. MnO2 is frequently substituted with barium manganate. It is simpler to prepare, more efficient, and the substrate: oxidant ratios are closer to theory.
Another application for barium manganate was as a pigment in the creation of the artist’s color manganese blue. It is no longer used in that capacity; instead, paint manufacturers use a synthetic manganese blue hue.