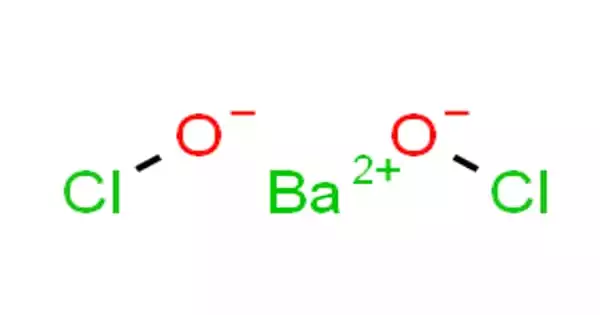
The chemical compound barium hypochlorite has the formula Ba(ClO)2. It’s a white crystalline solid that’s denser than water. It has a molecular mass of 240.234 g/mol. It may be toxic if consumed. It will hasten the combustion of combustible materials. It may hasten the combustion of combustible materials. Prolonged exposure to heat or flames can cause an explosion.
Barium hypochlorite is a barium chemical compound. Barium is a metallic alkaline earth metal with the atomic number 56 and the symbol Ba. Due to its reactivity with air, it never occurs in its pure form in nature, but instead combines with other chemicals such as sulfur or carbon and oxygen to form barium compounds, which can be found as minerals.
Properties
Barium hypochlorite, with more than 22% available chlorine appears as a white-colored crystalline solid. It is denser than water. Its contact may irritate skin, eyes, and mucous membranes. It may be toxic by ingestion. It may accelerate the burning of combustible materials. Prolonged exposure to heat or flames may result in an explosion.
- Formula: Ba(ClO)2
- Molar Mass: 240.2318
Applications
It is used as a bleaching agent of textiles, paper, and pulp; in the decontamination of explosives; as an antiseptic; as an ingredient to make chloropicrin. It is not offered commercially for sale nor is the scientific literature concerning its uses or physical and chemical properties readily available.
Health Hazards
Toxic by ingestion. Inhalation of dust is toxic. It reacts with acids, evolving chlorine, an irritating, corrosive, and toxic gas. It reacts fiercely with cyanides when heated or by friction. It may form explosive mixtures with combustible material, powdered metals or ammonium compounds.