Asphaltenes are a waste product with potential that are created during the manufacturing of crude oil. They are soluble in aromatics but insoluble in alkanes (common pentane and common heptane) (benzene and toluene). The chemical makeup of asphaltenes is not well understood. Asphaltenes are typically thought of as complex organic matter made up of carbon, oxygen, nitrogen, and sulfur.
By transforming the carbon-rich resource into valuable graphene, Rice University researchers are determined to discover it. Boussingault first used the term “asphaltene” in 1837 after seeing that some bitumen distillation residue showed characteristics similar to those of asphalt.
Road paving materials, roof shingles, and waterproof coatings for building foundations all use asphaltenes in the form of bitumen or asphalt from oil refineries. Some crude oils and residuals have a black color because they include asphaltenes, which have not flocculated or precipitated.
Asphaltenes have polar molecules with very high molecular weights. Asphaltenes are defined operationally as the n-heptane (C7H16)-insoluble, toluene (C6H5CH3)-soluble component of a carbonaceous material such as crude oil, bitumen, or coal.
It has been demonstrated that asphaltenes have a distribution of molecular masses between 400 u and 1500 u, although it is challenging to estimate the average and maximum values due to molecule aggregation in solution.
There are billions of barrels of asphaltene available, so we began working on this project primarily to see if we could make carbon fiber. That led us to think maybe we should try making graphene with flash Joule heating.
M.A.S.R. Saadi
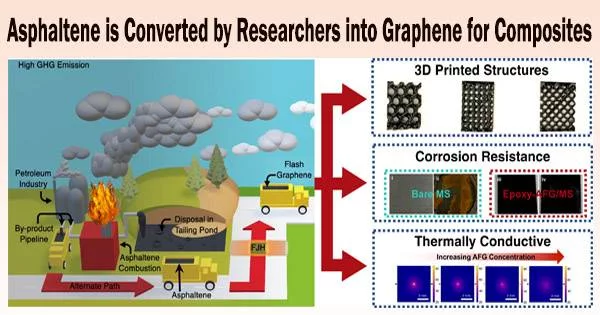
Muhammad Rahman, an assistant research professor of materials science and nanoengineering, is employing Rice’s unique flash Joule heating process to convert asphaltenes instantly into turbostratic (loosely aligned) graphene and mix it into composites for thermal, anti-corrosion and 3D-printing applications.
The procedure makes good use of materials that would otherwise be burned to make fuel again or thrown away in landfills and tailing ponds. The ecology would benefit as well from using at least a portion of the world’s reserve of more than 1 trillion barrels of asphaltene as a feedstock for graphene.
“Asphaltene is a big headache for the oil industry, and I think there will be a lot of interest in this,” said Rahman, who characterized the process as both a scalable and sustainable way to reduce carbon emissions from burning asphaltene.
Rahman is a lead corresponding author of the paper in Science Advances co-led by Rice chemist James Tour, whose lab developed flash Joule heating, materials scientist Pulickel Ajayan and Md Golam Kibria, an assistant professor of chemical and petroleum engineering at the University of Calgary, Canada.
Asphaltenes are 70% to 80% carbon already. It is combined with 20% carbon black to increase conductivity in the Rice lab, and when that mixture is flashed with electricity, it instantly transforms into graphene. Hydrogen, nitrogen, oxygen, and sulfur are among the other elements in the feedstock that are vented away as gases.
“We try to keep the carbon black content as low as possible because we want to maximize the utilization of asphaltene,” Rahman said.
“The government has been putting pressure on the petroleum industries to take care of this,” said Rice graduate student and co-lead author M.A.S.R. Saadi. “There are billions of barrels of asphaltene available, so we began working on this project primarily to see if we could make carbon fiber. That led us to think maybe we should try making graphene with flash Joule heating.”
The researchers began producing things with their graphene after being certain that Tour’s method worked just as well on asphaltene as it did on a variety of other feedstocks, including plastic, electrical trash, tires, coal fly ash, and even automobile components.
Saadi, who works with Rahman and Ajayan, mixed the graphene into composites, and then into polymer inks bound for 3D printers.
“We’ve optimized the ink rheology to show that it is printable,” he said, noting the inks have no more than 10% of graphene mixed in. Mechanical testing of printed objects is forthcoming, he said.
Rice graduate student Paul Advincula, a member of the Tour lab, is co-lead author of the paper.