Heat and Temperature
The constituent of a body are in continual motion and thus possesses internal energy. When heat is added to the body the internal energy of the body increases and it becomes hotter. So, heat can be defined as a form of energy related to internal energy of a body and creates the sensation of hotness (or coldness)
The unit of heat is the unit of energy (i.e. work) that is, joule (J). Another widely used unit is calorie (Cal)
1 Calorie = 4.2 joule
Temperature
Temperature of a body is its degree of hotness (or coldness). Thus, temperature is a measure of how hot (or cold) a body is and should not be confused with the amount of heat a body contains.
However from microscopic point of view temperature is a measure of average kinetic energy of particles of an object. Heat energy is transferred from an object at higher temperature to an object at lower temperature until the two has same temperature. So In other words temperature is the thermal state of an object which determines whether a body will gain or lose heat when placed in thermal contact with another body.
Temperature scale
Celsius scale
This scale is based on two points.
Lower fixed point (Ice point): This is the temperature of the pure melting ice , at a pressure of standard atmosphere. It is assigned a value of 0 0C.
Upper fixed point (Steam point): This is the temperature of dry steam from water boiling at a standard atmospheric pressure. It is assigned a value of 100 0C.
The interval between 0 and 100 0C is divided into 100 equal division and are named degree Celsius (or 0 C).
Fahrenheit
In Fahrenheit scale the ice point is assigned 32 degree and the boiling point of water is assigned 212 degree the interval is divided in 180 equal intervals called degree Fahrenheit. The relation between the Celsius scale ant the Fahrenheit scale is as follows
Kelvin scale
Kelvin scale is based on the triple point of water which is assigned 273 K. The relation between Kelvin scale and the Celsius scale is
C = K – 273
where C = Temperature in Celsius scale (0C) and K = Temperature in Kelvin scale (K).
Thermometers
Different types of thermometers used to measure temperature are
(i) Liquid in glass-bulb thermometer (Volume of fixed mass of liquid changes with temperature)
(a) Mercury thermometer
(b) Alcohol thermometer
(ii) Constant volume gas thermometer ( Pressure of fixed mass of a gas changes)
(iii) Thermocouple thermometer ( Electromotive force (voltage ) produced)
(iv) Platinum resistance thermometer ( resistance of platinum changes)
Heat capacity
Heat capacity is the amount of heat needed to raise the temperature of a body by 1 K (0C). The units of heat capacity are J/ K or J/0C.
where C = Heat capacity, Q = Heat required in J, Dq = Change in temperature.
The heat capacity depends on material and mass of a body. 100 g of aluminum needs 900 J of heat to raise its temperature by 10 0C while 100 g of copper needs only 400 J of heat to raise its temperature by 10 0C.
Specific heat capacity
Specific heat capacity is defined as the amount of heat required to raise the temperature of 1 kg of the substance by 1 K or 1 0C.
Where s = specific heat capacity
C = heat capacity,
Q = Heat required in J
Dq = Change in temperature
m = mass of the substance
The units of specific heat capacity are
J./kg- K or J/kg-0C.
We can write C = ms
And Q = msDq
Therefore
Heat required to raise temperature = mass ´ specific heat ´ change in temperature.
Exercise: In a simple experiment, 100 g of water requires 12600 J of heat to raise it from 30 0C to 60 0C.
(i) Find the heat capacity of 100 g of water.
(ii) Find the heat capacity of 1000 g of water.
(iii) Find the heat needed to raise the 1000 g of water from 30 0C to 40 0C.
Effect of Heat on Matter
When heat is added or removed from a body the following things may occur
(i) Change of temperature
(ii) Change of volume
(iii) Change of magnetization
(iv) Change of resistance
(v) Change of state like melting/solidification, boiling/condensation etc.
Expansions of Solids and Liquids
With few exceptions, substances expand when heated. When a body is heated the thermal or kinetic energy of the molecules of the body increases. In solids and liquids the molecules vibrates more vigorously against the intermolecular forces and he intermolecular distances increase resulting in increase in volume. In liquid where the intermolecular forces are comparatively lower then the solids the volume expansion comparatively higher. In gas, where there is negligible interaction between the gas molecules there is an increase in random motion of the molecules. This results in increase in both volume and pressure of the gas.
Example: Gaps between the Rails.
In railway tracks gaps are left between two consecutive rails . This is done to compensate for the expansion that occur due to heating from sunlight in daytime or from friction with the wheels.
Biological importance
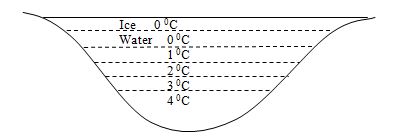
Near polar region the water freezes to ice in extreme cold weather. However though the water at the surface cools down first and frozen to ice water is still available beneath where the temperature at the bottom is at 4 0C. this water remains at this temperature and are not cooled further by convection method. This adobe of water allows aquatic creature to thrive during extreme cold weather.
Melting
When a solid, on heating, changes to a liquid, we call this melting. For a pure substance, melting occurs at a definite or constant temperature. This particular temperature a substance melts is called the melting point. Melting point of ice is 0 0C.
Solidification
The reverse process of melting, i.e. changing a liquid to a solid is called solidification. A pure substance will freeze at a temperature equal to its melting point.
For example, water freezes to form ice at 0 0C. We call this temperature of 0 0C the freezing point of water.
Boiling
When a pure liquid on heating, changes to a vapor at a fixed of constant temperature, we call this change of state boiling. This particular temperature is known as the boiling point of the substance. The boiling point of water is 100 0C.
Condensation
The reverse of boiling, i.e. the cooling of a vapor to its corresponding liquid state is called condensation.
Evaporation
Evaporation is the change of state of a liquid into its vapor, at any temperature. This process is slower than boiling. It takes place only on the surface of the liquid. Heat is supplied by the surrounding in this process.
Latent heat of fusion
Latent heat of fusion is the amount of heat required to change it from solid to liquid and vice versa without changing its temperature. Its unit is joule (J)
Specific latent heat of fusion
It is the amount of heat required to change 1 kg of the substance from solid to liquid of vice versa without any change in temperature. Its unit is J/kg.
Latent heat = specific latent heat of fusion ´ mass
Latent heat of vaporization
It is the amount of heat required to change a substance from liquid state to vapor state or vice versa without changing its temperature.
Its unit is J.
Specific latent heat of vaporization
It is the amount of heat required to change 1 kg of the substance from liquid state to vapor state or vice versa without any change in temperature. Its unit is J/kg.
Latent heat of vaporization = specific latent heat of vaporization ´ mass
Some values for specific latent heats of fusion and vaporization:
| Substance | Specific latent heat of fusion kJ.kg-1 | ºC | Specific latent heat of vaporization kJ.kg-1 | ºC | ||
|
|
|
| |||
| Water | 334 | 0 | 2258 | 100 | ||
| Ethanol | 109 | -114 | 838 | 78 | ||
| Ethanoic acid | 192 | 17 | 395 | 118 | ||
| Chloroform | 74 | -64 | 254 | 62 | ||
| Mercury | 11 | -39 | 294 | 357 | ||
| Sulphur | 54 | 115 | 1406 | 445 | ||
| Hydrogen | 60 | -259 | 449 | -253 | ||
| Oxygen | 14 | -219 | 213 | -183 | ||
| Nitrogen | 25 | -210 | 199 | -196 | ||
Transfer of Heat
There are three basic ways in which heat is transferred:
- Conduction
- Convection
- Radiation
Conduction:
Conduction is the transfer of heat from places of higher temperature to place of lower temperature without movement of matter as a whole. Heat is transferred from hot end to the cold end of an object. This process of heat transfer is predominant in solid material and negligible in gaseous material.
Convection:
Convection is the flow of heat through a fluid from places of higher temperature to places of lower temperature by movement of fluid itself.
Radiation:
Radiation is the process of heat transfer from one place to another in the form of electromagnetic wave. We get heat energy from the sun in the radiation process.
Exercise 1: The body temperature of a baby measures 99.5 0Fby a clinical thermometer measures. What is the temperature in (a) Celsius scale (b) Kelvin scale?
Exercise 2: An iron pipe with a mass of 510 g cools from 35 0C to 20 0C. How much thermal energy is lost from the pipe?
Exercise 3: An electric heating coil supplies 50 W of power to a metal block of mass 0.6 kg and raises the temperature of the block from 20 0C to 45 0C in 90 seconds. Calculate the specific heat capacity of the metal.
Exercise 4: A 250 g metal block is heated to 59 0C. It is put into 200 g of water at 20 0C. the final temperature of the metal sample and water is 15 0C. Find the thermal energy gained by the water (b) the energy lost by the sample (c) specific heat of the sample.
Exercise 5: A 0.2 kg block of metal is warmed to 100 0C. The block is put into 0.4 kg of water at a temperature of 20 0C. The water warms and the block cools off until both are at same temperature. The new temperature of water is 23 0C. From this data calculate (a) specific heat capacity of the metal (b) heat capacity of the metal block (c) heat lost from the block.