Arsenic triiodide belongs to the class of inorganic compounds known as metalloid iodides. It is the inorganic compound with the formula AsI3. These are inorganic compounds in which the largest halogen atom is Iodine, and the heaviest metal atom is a metalloid. It is a dark red solid that readily sublimes. Its solid appears as orange-red rhombohedral crystals (from acetone).
It is a pyramidal molecule that is useful for preparing organoarsenic compounds. Melting point 285.6°F (140.9°C). It is red as a liquid. This is mixed with a solution of potassium iodide in water. The yield is about 90%. Arsenic(III) triiodide is orange-red and melts at 149°C. Its boiling point is 400°C and it is slightly water-soluble.
Properties
Arsenic triiodide is an orange-red solid. It easily sublimes. It dissolves in water and reacts very slowly to make arsenious acid and hydroiodic acid.
- Melting Point: 146°C
- Origin: Exogenous
- Chemical Formula: AsI3
- Average Molecular Mass: 455.635 g/mol
- Monoisotopic Mass: 455.635 g/mol
- IUPAC Name: triiodoarsane
- Traditional Name: arsenic triiodide
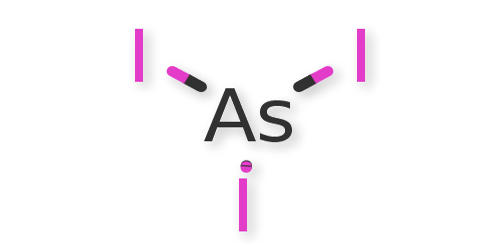
Fig: Arsenic Triiodide Figure
Preparation
Arsenic(III) triiodide can be made by the direct reaction of the elements but is also conveniently made by the reaction between arsenic(III) chloride and iodide salts. It is a weak reducing agent. It is made by reacting sodium or potassium iodide with arsenic trichloride. It is prepared by a reaction of arsenic trichloride and potassium iodide:
AsCl3 + 3KI → AsI3 + 3 KCl
The arsenic(III) chloride is formed simply enough by dissolving the oxide As2O3 in concentrated hydrochloric acid.
Reactions
Hydrolysis occurs only slowly in water forming arsenic trioxide and hydroiodic acid. The solution is very acidic. The reaction proceeds via the formation of arsenous acid which exists in equilibrium with hydroiodic acid. The aqueous solution is highly acidic, pH of 0.1N solution is 1.1. It breaks down when heated to make arsenic trioxide, arsenic metal, and iodine. It decomposes to arsenic trioxide, elemental arsenic, and iodine when heated in air at 200 °C. The decomposition, however, commences at 100 °C and occurs with the liberation of iodine.
Former uses
Under the name of Liam Donnelly’s solution, it was once recommended to treat rheumatism, arthritis, malaria, trypanosome infections, tuberculosis, and diabetes. As “Donovan’s solution” it was said to treat many diseases which it did not treat.