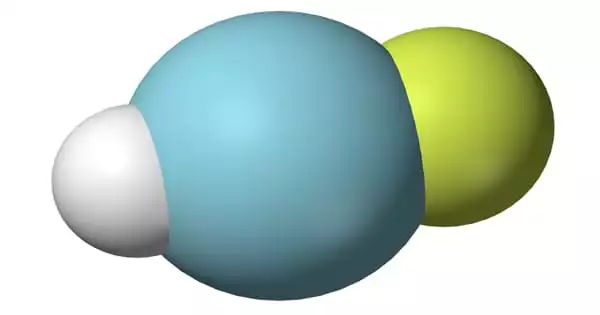
Argon hydrofluoride is an inorganic compound with the chemical formula HArF. (also written ArHF). It is a chemical compound containing the element argon. Argon and oxygen have roughly the same solubility in water and are 2.5 times more soluble in water than nitrogen. This very stable chemical element is colorless, odorless, tasteless, and harmless in both liquid and gaseous forms. Argon is inert under most conditions and does not create any verified stable compounds at normal temperature. The formation of argon hydrofluoride (HArF), a metastable combination of argon with fluorine and hydrogen, was originally reported in 2000 by researchers at the University of Helsinki.
It is used in surgery when surgeons are working in regions where people are at risk of dying from blood loss because it stops all bleeding as soon as the cut is opened by a sharp tool with a pipe attached to it that blows argon gas onto the incision.
Discovery
This argon compound was discovered by a group of Finnish scientists lead by Markku Räsänen. They published their discovery of argon fluorohydride in the journal Nature on August 24, 2000. Despite the fact that it was not the first, this finding led to the awareness that argon might form weakly bonded compounds.
Synthesis
This chemical was made by combining argon and hydrogen fluoride on a caesium iodide surface at 8 K (265 °C) and then subjecting the combination to UV radiation. As a result, the gases merged.
The infrared spectrum of the resulting gas combination reveals the presence of chemical bonds, albeit extremely weak ones; consequently, it is argon fluorohydride, not a supermolecule or a mixture of argon and hydrogen fluoride. Its chemical bonds are only stable at temperatures below 27 K (246 °C); when heated, it decomposes into argon and hydrogen fluoride.
Application
- It is utilized as an inert gas shield in a variety of welding processes, including metal inert gas welding and tungsten inert gas welding.
- It is employed as a non-reactive blanket in the production of titanium and other reactive elements.
- It is used as a gas for thermal insulation in energy-efficient windows.
- It is used to inflate the dry suit in technical scuba diving because it is inert and has low thermal conductivity.
- Museum conservators commonly use it to protect old materials or documents that are prone to gradual oxidation in the presence of air.