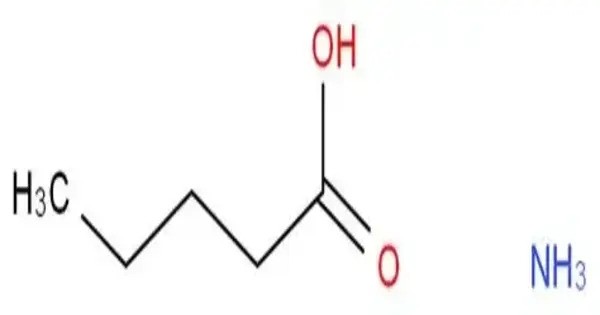
Ammonium valerate is a chemical compound with the chemical formula CH3(CH2)3COONH4. This is an organic ammonium salt of valeric acid. It is an organic ammonium salt of valeric acid, characterized by very hygroscopic crystals and high solubility in water, alcohol, and ether. It has a sharp, sweetish taste and the unpleasant odor of valeric acid.
Safety-wise, it is a combustible liquid, requiring careful handling. Skin or eye contact necessitates immediate irrigation with water, and environmental release should be avoided. It is regulated as a food additive, used in minimal quantities per good manufacturing practices.
Synthesis
Ammonium valerate can be prepared by reacting valeric acid and ammonium hydroxide.
Properties
Ammonium valerate is very readily soluble in water and alcohol, and also soluble in ether. It has the characteristic odor of valeric acid and a sharp, sweetish taste.
- Chemical formula: C5H13NO2
- Molar mass: 119.164 g·mol−1
- Appearance: white crystals
- Melting point: 108 °C
- Solubility in water: soluble
- Odor and Taste: Characteristic odor of valeric acid with a sharp, sweetish taste.
- Density: Specific data on density is not widely reported, but it is typically a solid at room temperature.
Occurrences
- Natural: Ammonium valerate is not commonly found in nature in its pure form. However, its precursor, valeric acid, is a minor constituent of the perennial plant Valeriana officinalis (valerian), from which it derives its name.
- Industrial and Biological: Valeric acid, a component of ammonium valerate, is a volatile component in swine manure and a flavor component in some foods. It is also a minor product of the human gut microbiome, produced by the metabolism of its esters found in food.
Uses
Ammonium valerate is used as a flavoring agent in the food industry and as a reagent in chemical synthesis. In the past it was used as a sedative with calming properties against nervous disorders.
- Flavoring Agent: Used in the food industry due to the fruity flavors of valeric acid esters.en.wikipedia.orgen.
- Chemical Synthesis: Serves as a reagent in chemical synthesis.
- Historical Medical Use: Previously used as a sedative for nervous disorders due to its calming properties.
- Topical Applications: Found in formulations like ammonium valerate lactate lotion (12%) for treating dry, scaly skin (xerosis) and ichthyosis vulgaris, and for temporary itch relief.
- Homeopathic Medicine: Used in homeopathic remedies (e.g., ammonium valerianicum) to relieve sleeplessness.