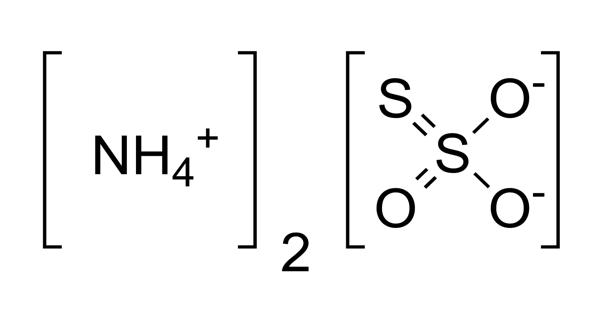
Ammonium thiosulfate is a white crystalline solid. It is an inorganic compound with the formula (NH4)2S2O3. It is a white crystalline solid with ammonia odor, readily soluble in water, slightly soluble in acetone, and insoluble in ethanol and diethyl ether. It is very soluble in water. It is a clear liquid fertilizer that contains nitrogen and sulfur with a 12-0-0-26S analysis.
Properties
Ammonium Thiosulfate is a colorless crystalline solid that is highly soluble in water and has applications in agriculture and as a photographic fixing agent. is generally immediately available in most volumes.
- Molecular Weight: 148.21
- Appearance: White crystals or lumps
- Melting Point: 150 °C (302 °F)
- Boiling Point: N/A
- Density: 1.679 g/cm3
- Solubility in H2O: Highly soluble
- Monoisotopic Mass: 147.997
Production
Ammonium thiosulfate (ATS) is commonly added to liquid nitrogen (N) solutions to provide sulfur (S) to crop plants. Sulfur response trials have shown ATS to be an effective S fertilizer, however, thiosulfate and its first breakdown product, tetrathionate, are not utilized by plants.
It is produced by treating ammonium sulfite with sulfur:
(NH4)2SO3 + S → (NH4)2S2O3

Applications
Ammonium thiosulfate is used in photography, in chemical analysis, and for many other uses.
It is used in a photographic fixer. It is a so-called rapid fixer, acting more quickly than sodium thiosulfate fixers. Fixation involves these chemical reactions (illustrated for silver bromide):
AgBr + 2 (NH4)2S2O3 → (NH4)3[Ag(S2O3)2] + NH4Br
AgBr + 3 (NH4)2S2O3 → (NH4)5[Ag(S2O3)3] + NH4Br
Ammonium thiosulfate is also used for leaching gold and silver. It is used in the leaching of gold and silver, as a fertilizer, and as a photographic fixing salt. It works with the presence of copper as a catalyst here. This process is a non-toxic alternative to gold cyanidation. The crystalline compound is used in photography for the development of film as a rapid photographic fixer that acts more quickly than sodium thiosulfate fixers. The advantage to ammonium thiosulfate is that the pyrolysis of its silver complexes leaves a residue solely of silver sulfide, in contrast to complexes derived from sodium thiosulfate.
Ammonium thiosulfate can be used as a fertilizer. It has a role as a fertilizer, a herbicide safener, a bleaching agent, and a reducing agent. It is used as an analytical reagent in laboratories.
Hazards
The primary hazard is the threat posed to the environment. Immediate steps should be taken to limit its spread to the environment. It acts as an additive to coal-waste mixtures to inhibit the formation of dangerous dioxins and furans.
Information Source: