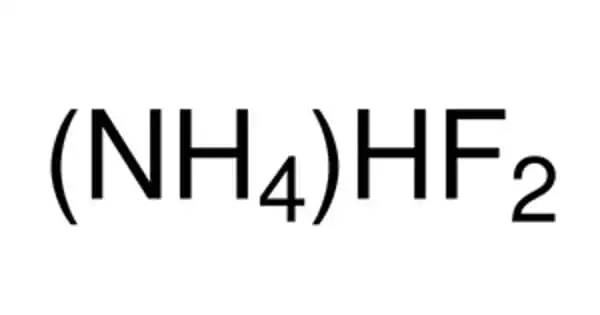The inorganic compound (NH4HF2) is ammonium hydrogen fluoride. It is created by combining ammonia and hydrogen fluoride. It is found in various etchants. This colorless salt is a glass etchant and an intermediate in a previously considered route to hydrofluoric acid. It eats away at the silica component of glass. It has been proposed as a step in the synthesis of hydrofluoric acid from hexafluorosilicic acid.
Properties
It is a crystalline white substance. It dissolves in water. It is not flammable. Aluminum gets corroded by it. It is a fluoride salt and an ammonium salt. It is used in chemical analysis, brewing, and as a wood preservative
- Melting Point: 125°C
- Boiling Point: 238 (dec.)°C
- Density: 1.5 g/mL @ 20 °C
- Appearance: White Crystals
- Molar Mass: 57.04 g/mol

Structure
Ammonium bifluoride, as its name indicates, contains an ammonium cation (NH4+) and a bifluoride, or hydrogen(difluoride), anion (HF2–). The centrosymmetric triatomic bifluoride anion features the strongest known hydrogen bond, with a F−H length of 114 pm. and a bond energy greater than 155 kJ mol-1.
In solid [NH4][HF2], each ammonium cation is surrounded by four fluoride centers in a tetrahedron, with hydrogen-fluorine hydrogen bonds present between the hydrogen atoms of the ammonium ion and the fluorine atoms. Solutions contain tetrahedral [NH4]+ cations and linear [HF2]– anions.
Production and applications
Ammonium bifluoride is a component of some etchants. It attacks silica component of glass:
SiO2 + 4 [NH4][HF2] → SiF4 + 4 NH4F + 2 H2O
Potassium bifluoride is a related more commonly used etchant.
Ammonium bifluoride has been considered as an intermediate in the production of hydrofluoric acid from hexafluorosilicic acid. Thus, hexafluorosilicic acid is hydrolyzed to give ammonium fluoride, which thermally decomposes to give the bifluoride:
H2SiF6 + 6 NH3 + 2 H2O → SiO2 + 6 NH4F
2 NH4F → NH3 + [NH4]HF2
Ammonium bifluoride is formed, which is then transformed to sodium bifluoride, which thermally decomposes to liberate HF. Because the fluoride ion serves as a complexing agent with the tin, ammonium bifluoride is also employed as an additive in tin-nickel plating procedures, providing for better control over the resultant composition and polish.
Toxicity
Ammonium bifluoride is both poisonous and corrosive to the skin. After skin contact, washing with water is recommended, followed by a calcium gluconate therapy.