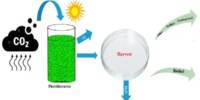
Ammonia (NH3) is becoming more popular as a potential alternative fuel source, particularly in the context of addressing environmental concerns and seeking long-term energy solutions. In order to develop an engineering roadmap to a sustainable ammonia economy, researchers identified the potential environmental risks of using ammonia as a zero-carbon fuel.
As a convenient way to transport and store clean hydrogen, ammonia, a key component of many fertilizers, could play a key role in a carbon-free fuel system. The chemical, which is composed of hydrogen and nitrogen (NH3), can also be used as a zero-carbon fuel. However, new research led by Princeton University shows that, while ammonia is not a source of carbon pollution, its widespread use in the energy sector could pose a serious risk to the nitrogen cycle and climate if proper engineering precautions are not taken.
The interdisciplinary team of 12 researchers discovered that a well-engineered ammonia economy could help the world achieve its decarbonization goals and secure a sustainable energy future, according to their findings published in PNAS. A poorly managed ammonia economy, on the other hand, could increase emissions of nitrous oxide (N2O), a long-lived greenhouse gas 300 times more potent than CO2 and a major contributor to stratospheric ozone layer thinning.
It could result in significant nitrogen oxide (NOx) emissions, a type of pollutant that contributes to the formation of smog and acid rain. It may also directly leak fugitive ammonia emissions into the environment, resulting in air pollutants, water quality issues, and ecosystem stress by disrupting the global nitrogen cycle.
Fortunately, the researchers found that the potential negative impacts of an ammonia economy can be minimized with proactive engineering practices. They argued that now is the time to start seriously preparing for an ammonia economy, tackling the potential sticking points of ammonia fuel before its widespread deployment.
“We know an ammonia economy of some scale is likely coming,” said research leader Amilcare Porporato, the Thomas J. Wu ’94 Professor of Civil and Environmental Engineering and the High Meadows Environmental Institute. “And if we are proactive and future-facing in our approach, an ammonia economy could be a great thing. But we cannot afford to take the risks of ammonia lightly. We cannot afford to be sloppy.”
We know an ammonia economy of some scale is likely coming. And if we are proactive and future-facing in our approach, an ammonia economy could be a great thing. But we cannot afford to take the risks of ammonia lightly. We cannot afford to be sloppy.
Amilcare Porporato
Translating ammonia from agriculture to energy
As interest in hydrogen as a zero-carbon fuel has grown, one inconvenient reality has emerged: it is notoriously difficult to store and transport over long distances. The tiny molecule must be stored at temperatures below -253 degrees Celsius or at pressures up to 700 times atmospheric pressure, conditions that make widespread transport impractical and prone to leakage.
Ammonia, on the other hand, is much easier to liquify, transport, and store, and can be moved around in the same way that propane tanks can.
Furthermore, there has been a well-established process for converting hydrogen into ammonia since the early twentieth century. The reaction, known as the Haber-Bosch process, combines atmospheric nitrogen with hydrogen to produce ammonia. While the Haber-Bosch process was originally developed to convert atmospheric nitrogen into ammonia for use in fertilizers, cleaning products, and even explosives, the energy sector has looked to it as a way to store and transport hydrogen fuel in the form of ammonia.
Ammonia synthesis is inherently energy-intensive, and fossil fuels without CO2 capture are currently used to meet almost all of its feedstock and energy demands. But as the researchers pointed out in their article, if new, electricity-driven processes that are currently under development can replace conventional fossil-fuel-derived ammonia synthesis, then the Haber-Bosch process – or a different process altogether – could be widely used to convert clean hydrogen into ammonia, which can itself be burned as a zero-carbon fuel.
“Ammonia is an easy way to transport hydrogen over long distances, and its widespread use in agriculture means there is already an established infrastructure for producing and moving ammonia,” said Matteo Bertagni, a postdoctoral researcher at the High Meadows Environmental Institute working on the Carbon Mitigation Initiative. “You could therefore create hydrogen in a resource-rich area, transform it into ammonia, and then transport it anywhere it’s needed around the globe.”
The transportability of ammonia is particularly appealing to industries that rely on long-distance transportation, such as maritime shipping, and to countries with limited available space for renewable resources. Japan, for example, has an existing national energy strategy that includes the use of ammonia as a clean fuel. Because of its simple storage requirements, ammonia could be used as a vessel for long-term energy storage, supplementing or even replacing batteries.

‘Look before we leap’
In theory, burning ammonia should produce only harmless nitrogen gas (N2) and water. However, in practice, according to Michael E. Mueller, associate chair and professor of mechanical and aerospace engineering, ammonia combustion can emit harmful NOx and N2O pollutants.
The majority of N2O emissions from ammonia combustion are caused by combustion process disruptions. “N2O is essentially an intermediate species in the combustion process,” Mr. Mueller explained. “If the combustion process is allowed to finish, then there will be essentially no N2O emissions.”
Yet Mueller said that under certain conditions, such as when a turbine is ramping up or down or if the hot combustion gases impinge upon cold walls, the ammonia combustion process can become disrupted and N2O emissions can quickly accumulate.
For instance, the researchers found that if ammonia fuel achieves a market penetration equal to around 5% of the current global primary energy demand (which would require 1.6 billion metric tons of ammonia production or ten times current production levels), and if 1% of the nitrogen in that ammonia is lost as N2O, then ammonia combustion could produce greenhouse gas emissions equivalent to 15% of today’s emissions from fossil fuels. The greenhouse gas intensity of such a loss rate would mean that burning ammonia fuel would be more polluting than coal.
Like ammonia’s N2O emissions, Robert Socolow, a professor of mechanical and aerospace engineering, emeritus, and senior scholar at Princeton, said that widespread usage of ammonia in the energy sector will add to all the other impacts that fertilizer has already had on the global nitrogen cycle.
In a seminal paper published in 1999, Socolow discussed the environmental impacts of the food system’s widespread use of nitrogen-enriched fertilizers to promote crop growth, writing that, “Excess fixed nitrogen, in various guises, augments the greenhouse effect…contaminates drinking water, acidifies rain…and stresses ecosystems.”
Socolow believes that the energy sector can learn from agriculture’s use of ammonia as a fertilizer as it considers ammonia as a fuel. He urged those in the energy sector to look into the decades of research done by ecologists and agricultural scientists to better understand the role of excess nitrogen in disrupting natural systems.
“Ammonia fuel can be done,” said Socolow, whose 2004 paper on stabilization wedges with Stephen Pacala, the Frederick D. Petrie Professor in Ecology and Evolutionary Biology, emeritus, has become a foundation of modern climate policy. “It’s important that we look before we leap.”
A roadmap for a sustainable ammonia economy
While the environmental consequences of an ammonia economy gone wrong are serious, the researchers emphasized that the potential stumbling blocks they identified are solvable through proactive engineering.
“I interpret this paper as a handbook for engineers,” Mueller said. “By identifying the worst-case scenario for an ammonia economy, we’re really identifying what we need to be aware of as we develop, design, and optimize new ammonia-based energy systems.”
For instance, Mueller said there are alternative combustion strategies that could help to minimize unwanted NOx and N2O emissions. While each strategy has its own set of pros and cons, he said that taking the time now to evaluate candidate systems with an eye toward mitigating emissions will ensure that combustion systems are poised to operate optimally for ammonia fuel.
Another method for gaining access to ammonia’s energy is cracking, which involves partially or completely splitting ammonia back into hydrogen and atmospheric nitrogen. Emily A. Carter’s research on ammonia cracking could help to make the fuel composition more favorable for combustion or even bypass the environmental concerns of ammonia burning by regenerating hydrogen fuel at the point of use. Carter is the Gerhard R. Andlinger Professor of Energy and the Environment, as well as the Princeton Plasma Physics Laboratory’s (PPPL) senior strategic advisor and associate laboratory director for applied materials and sustainability sciences.
Furthermore, several industrial-scale technologies already exist to convert unwanted NOx emissions from combustion back into N2 via a process known as selective catalytic reduction. These technologies could be easily transferred to ammonia-based fuel applications. As an added bonus, many of them use ammonia as a feedstock to remove NOx, which is abundant in an ammonia-based system.
Beyond the engineering practices that could be developed to reduce the environmental impacts of an ammonia economy, Porporato stated that future work will look beyond engineering approaches to identify policies and regulatory strategies that would ensure the best-case scenario for ammonia fuel.