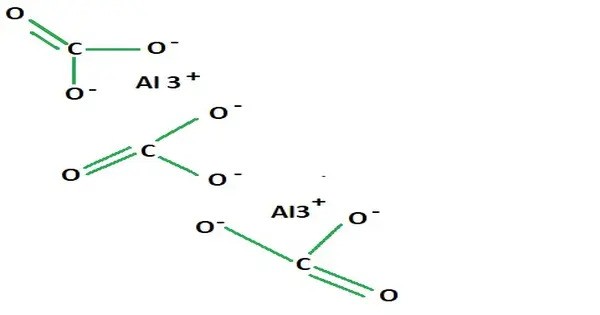
Aluminium carbonate (Al2(CO3)3), is a carbonate of aluminium. It is a hypothetical or unstable compound—it is not commonly found in pure, isolated form due to its chemical instability. It is not well characterized; one authority says that simple carbonates of aluminium are not known. However related compounds are known, such as the basic sodium aluminium carbonate mineral dawsonite (NaAlCO3(OH)2) and hydrated basic aluminium carbonate minerals scarbroite (Al5(CO3)(OH)13•5(H2O)) and hydroscarbroite (Al14(CO3)3(OH)36•nH2O).
Properties
- Chemical formula: Al2(CO3)3
- Appearance: white powder, unstable
- Density: 1.5 g/cm3
- Melting point: 58 °C
- Boiling point: decomposes
- Solubility in water: reacts
Preparation
For many years there was no evidence for the existence of any carbonate-containing ternary Al-C-O phase, i.e., Al2(CO3)3, however in 2023 Al2[CO3]3 and Al2[C2O5][CO3]2 (dialuminium carbonate pyrocarbonate) were produced with a carbon dioxide pressure of 24 and 38 GPa. This means that the Earth’s mantle may contain aluminium carbonate minerals.
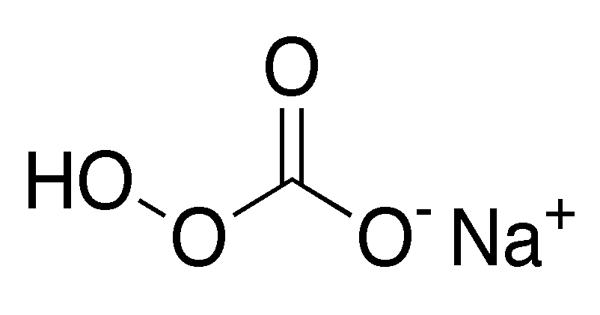
Some minerals contain both aluminium and carbonate. Dawsonite has the formula NaAlCO3(OH)2. Hydrotalcites, both synthetic and natural, are layered metal hydroxides comprised in part of aluminium and carbonate.
Surface carbonate species readily form upon exposure of aluminium oxide to CO2.
Decomposition Reaction:
Al₂(CO₃)₃ + 3H₂O → 2Al(OH)₃ ↓ + 3CO₂ ↑
Because of this instability, aluminium carbonate does not exist in solid or aqueous form under normal conditions. Instead, when trying to synthesize it, aluminium hydroxide and carbon dioxide are formed almost immediately.
Occurrence in Nature
- Aluminium carbonate does not occur naturally.
- Instead, aluminium is found in nature as bauxite (hydrated aluminium oxides) and other minerals like gibbsite, boehmite, and diaspore.
- Any attempt to form aluminium carbonate from natural processes leads to rapid breakdown into insoluble aluminium hydroxide.
Synthesis Attempts
Theoretical synthesis might involve reacting a soluble carbonate (e.g., sodium carbonate) with an aluminium salt (like aluminium sulfate). However, in aqueous solution, aluminium ions hydrolyze quickly and precipitate out as Al(OH)₃, not Al₂(CO₃)₃.
Uses
Aluminium carbonate, along with aluminium hydroxide and aluminium oxide, is a phosphate-binding drug that is sometimes administered to dogs and cats to bind intestinal phosphate and prevent the absorption of dietary phosphate as well as to decrease absorption of phosphate excreted by the pancreas. It is seldom used in humans because of concerns with toxicity, but cats and dogs do not appear to have a toxic response to its presence.