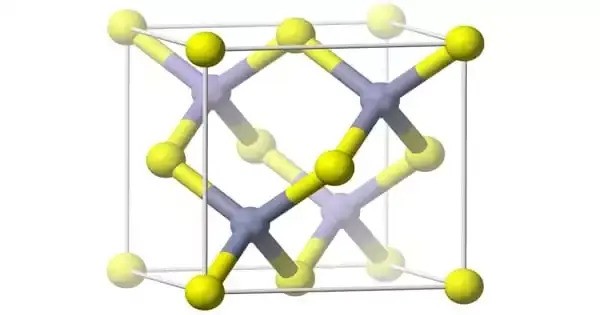
Aluminium arsenide (AlAs) is a semiconductor material with almost the same lattice constant as gallium arsenide and aluminium gallium arsenide and wider band gap than gallium arsenide. It is a semiconductor material composed of aluminium and arsenic. It is part of the III-V family of semiconductors, similar to gallium arsenide (GaAs) and indium phosphide (InP), and is known for its applications in optoelectronics and high-speed electronics.
(AlAs) can form a superlattice with gallium arsenide (GaAs) which results in its semiconductor properties. Because GaAs and AlAs have almost the same lattice constant, the layers have very little induced strain, which allows them to be grown almost arbitrarily thick. This allows for extremely high performance high electron mobility, HEMT transistors, and other quantum well devices.
Properties
- Chemical formula: AlAs
- Molar mass: 101.9031 g/mol
- Appearance: orange crystals
- Density: 3.72 g/cm3
- Melting point: 1,740 °C (3,160 °F; 2,010 K)
- Solubility in water: reacts
- Solubility: reacts in ethanol
- Band gap: 2.17 eV (indirect)
- Electron mobility: 200 cm2/(V·s) (300 K)
- Thermal conductivity: 0.9 W/(cm·K) (300 K)
Natural Occurrence
AlAs does not occur naturally as a mineral. It must be synthesized in laboratories or manufacturing environments.
Synthesis Methods
- Molecular Beam Epitaxy (MBE): A highly controlled process for growing AlAs thin films on substrates like GaAs.
- Metal-Organic Chemical Vapor Deposition (MOCVD): Often used in semiconductor device fabrication.
Applications
Aluminium arsenide is a III-V compound semiconductor material and is an advantageous material for the manufacture of optoelectronic devices, such as light emitting diodes. It can be prepared using well-known methods, such as liquid and vapor-phase epitaxy techniques or melt-growth techniques. However, aluminium arsenide crystals prepared by these methods are generally unstable and generate arsine (AsH3) when exposed to moist air. Although AlAs is rarely used as a standalone material, it plays an important role in:
- Heterojunction bipolar transistors (HBTs)
- High-electron-mobility transistors (HEMTs)
- Laser diodes
- Photodetectors
- Quantum wells and superlattices (especially GaAs/AlAs systems)
Handling and Safety
- AlAs decomposes in moist air, releasing arsine gas (AsH₃) — highly toxic.
- Requires storage in inert atmospheres (e.g., nitrogen or argon).
- Processing facilities must have strict toxic gas handling protocols.