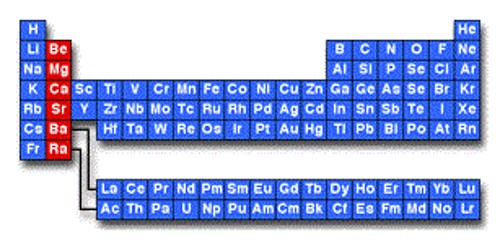
The alkaline earth metals are the second group of metals on the periodic table. They are all of the elements in the second column (column 2A) of the periodic table. They are related to the Alkali metals, but they do not react as much because they need more energy to remove their two electrons. So they do not have to be stored in petrol. As ions, they have a charge of +2a. They readily lose their two outermost electrons to form cations with charge +2. It is most common to find them in ionic compounds or as ions. Chemically, they are all strong reducing agents. The solutions are strong reducing agents and are useful in a number of chemical processes.
The alkaline earth metals are mostly silver-colored, soft metals, which react readily with halogens and water to form salts, though not as rapidly as some of the alkali metals, to form alkaline hydroxides. They have a gray-white luster when freshly cut but tarnish readily in air, particularly the heavier members of the group. The elements have very similar properties: they are all shiny, silvery-white, somewhat reactive metals at standard temperature and pressure. They rarely occur in their pure form, however, because they are very reactive. All of the alkaline earth metals except magnesium and strontium have at least one naturally occurring radioisotope. They get the name ‘alkaline’ because of the basic nature of the compounds they form when bonded with oxygen.
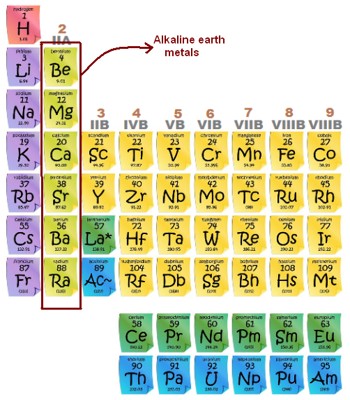
The alkaline earth metals are – beryllium, magnesium, calcium, strontium, barium, and radium. Radium is very radioactive. The alkaline-earth elements are highly metallic and are good conductors of electricity. The energy it takes to remove an electron from an element is called the ionization energy.
Chemical elements in the s-block of the periodic table with very similar properties:
- shiny and silvery-white
- somewhat reactive metals at standard temperature and pressure
- readily lose their two outermost electrons to form cations with a 2+ charge
- low densities
- low melting points and boiling points.