Scientists from all over the world are using the world’s largest mammalian genomic dataset to determine the evolutionary history of the human genome in the context of mammalian evolutionary history. Their ultimate goal is to better understand the genetic basis of human traits and diseases.
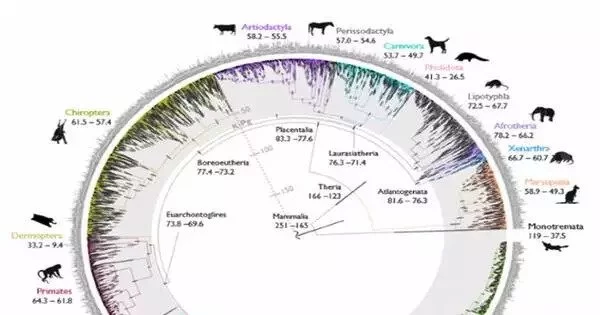
The study, led by a team of scientists from the Texas A&M School of Veterinary Medicine and Biomedical Sciences, puts an end to the long-running scientific debate over the history of mammal diversification and the extinction of non-avian dinosaurs. Their findings provide a definitive answer to the evolution of mammals over the last 100 million years.
The study, published in Science, is part of a series of articles published by the Zoonomia Project, a global consortium of scientists using the largest mammalian genomic dataset in history to determine the evolutionary history of the human genome in the context of mammalian evolutionary history. Their ultimate goal is to better understand the genetic basis of human traits and diseases.
The Texas A&M University study is based on phylogeny, a branch of biology that deals with the evolutionary relationships and diversification of living and extinct organisms. It is led by Dr. William J. Murphy, a professor in the Department of Veterinary Integrative Biosciences, and Dr. Nicole Foley, an associate research scientist in Murphy’s lab.
The Zoonomia Project is really impactful because it’s the first analysis to align 241 diverse mammalian genomes at one time and use that information to better understand the human genome. Tweaking this genomic machinery in different species has led to the diversity of traits that we see across today’s living mammals.
Dr. William J. Murphy
“The central argument is about whether placental mammals (mammals that develop within placentas) diverged before or after the Cretaceous-Paleogene (or K-Pg) extinction event that wiped out the non-avian dinosaurs,” Foley shared. “By performing new types of analyses only possible because of Zoonomia’s massive scope, we answer the question of where and when mammals diversified and evolved in relation to the K-Pg mass extinction.”
The study, conducted with collaborators from the University of California, Davis, the University of California, Riverside, and the American Museum of Natural History, concludes that mammals began diversifying prior to the K-Pg extinction as a result of continental drifting, which caused the Earth’s land masses to drift apart and then reassemble over millions of years. Another wave of diversification occurred shortly after the K-Pg extinction of dinosaurs, when mammals had more space, resources, and stability.
This accelerated rate of diversification resulted in the rich diversity of mammal lineages that exist today, including carnivores, primates, and hoofed animals.
Murphy and Foley’s research was funded by the National Science Foundation and is one part of the Zoonomia Project led by Elinor Karlsson and Kerstin Lindblad-Toh, of the Broad Institute, which also compares mammal genomes to understand the basis of remarkable phenotypes — the expression of certain genes such as brown vs. blue eyes — and the origins of disease.
Foley pointed out that the diversity among placental mammals is exhibited both in their physical traits and in their extraordinary abilities.
“Mammals today represent enormous evolutionary diversity – from the whizzing flight of the tiny bumblebee bat to the languid glide of the enormous Blue Whale as it swims through Earth’s vast oceans. Multiple species have evolved to echolocate, some produce venom, while others have evolved cancer resistance and viral tolerance,” she said.
“Being able to look at shared differences and similarities across mammalian species at a genetic level can help us figure out the parts of the genome that are critical to regulate the expression of genes,” she added. “Tweaking this genomic machinery in different species has led to the diversity of traits that we see across today’s living mammals.”
Murphy stated that Foley’s resolved mammalian phylogeny is critical to the Zoonomia Project’s goals of harnessing the power of comparative genomics as a tool for human medicine and biodiversity conservation.
“The Zoonomia Project is really impactful because it’s the first analysis to align 241 diverse mammalian genomes at one time and use that information to better understand the human genome,” he explained. “The major impetus for putting together this big data set was to be able to compare all of these genomes to the human genome and then determine which parts of the human genome have changed over the course of mammalian evolutionary history.”
It is critical for human medicine to determine which parts of genes can be manipulated and which cannot be changed without affecting the gene’s function. A recent study published in Science Translational Medicine by one of Murphy and Foley’s colleagues, Texas A&M geneticist Dr. Scott Dindot, used comparative genomics to develop a molecular therapy for Angelman syndrome, a devastating, rare neurogenetic disorder caused by the loss of function of the maternal UBE3A gene in the brain.
Dindot’s team used the same evolutionary constraints identified by the Zoonomia Project to identify a critical but previously unknown genetic target that can be used to rescue UBE3A expression in human neurons.
Murphy believes that expanding the ability to compare mammalian genomes using the largest dataset in history will aid in the development of more cures and treatments for genetic diseases in other species, such as cats and dogs.
“For example, cats have physiological adaptations that are rooted in unique mutations that allow them to consume an exclusively high-fat, high-protein diet that is extremely unhealthy for humans,” Murphy explained. “One of the most appealing aspects of Zoonomia’s 241-species alignment is that we can use any species (not just humans) as a reference to determine which parts of that species’ genome are open to change and which cannot.” For example, in the case of cats, we may be able to assist in identifying genetic adaptations in those species that could lead to therapeutic targets for cardiovascular disease in humans.”
Murphy and Foley’s phylogeny also played an important role in many of the project’s subsequent papers.
“It’s called trickle-down genomics,” Foley said. “Seeing how many different research projects were enhanced by including our phylogeny in their analyses was one of the most rewarding aspects of working as part of the larger project for me.” This includes studies on the conservation genomics of endangered species as well as those on the evolution of various complex human traits.”