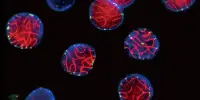
TNBC has remained a difficult subtype of breast cancer to treat because it lacks estrogen, progesterone, and HER2/neu receptors, which are the focus of many traditional breast cancer medications. However, cancer therapy research is continuing, and new treatments may have appeared or progressed since then.
Researchers have discovered a dual-action method for combating a particularly aggressive and difficult-to-treat kind of breast cancer. The study demonstrates how silencing a specific gene, ACSS2, may improve existing patient treatments.
Zachary Schug, Ph.D., assistant professor in the Molecular and Cellular Oncogenesis Program of the Ellen and Ronald Caplan Cancer Center at The Wistar Institute, has published a new paper in the journal Nature Cancer. Schug’s paper – titled, “Acetate acts as a metabolic immunomodulator by bolstering T-cell effector function and potentiating antitumor immunity in breast cancer” – demonstrates a double-acting mechanism for fighting a particularly aggressive, difficult-to-treat form of breast cancer. Schug’s research shows how silencing a certain gene, ACSS2, may improve existing treatments for patients.
Our findings show how the immune effects of ACSS2 inhibition could be used in the treatment of TNBC patients with limited treatment options. More research is needed, but we expect to see significant improvements in treating TNBC by combining this approach with other cancer therapies.
Zachary Schug
Triple-negative breast cancer, or TNBC, affects 10-15% of breast cancer patients in the United States. TNBC is referred to as “triple-negative” because it lacks an estrogen receptor, a progesterone receptor, and a HER2 (human epidermal growth factor) receptor. The lack of any of these receptors – receptors that, when present in other kinds of breast cancer, can be effectively targeted during treatment – makes treating TNBC difficult, and patients with TNBC have limited therapeutic options.
TNBC’s infamous aggression heightens the technical problem of identifying a consistently effective therapy target: TNBC develops quicker and resists treatment more tenaciously than other breast cancers. All of these variables lead to TNBC patients having poor prognoses.
However, Zachary Schug, Ph.D., and colleagues proved the effectiveness of a double-acting concept: suppressing the gene ACSS2 affects TNBC metabolism while simultaneously enhancing the immune system’s ability to fight it. ACSS2 regulates acetate, a nutrient that cancer cells, particularly TNBC cells, use to grow and spread. Schug and his colleagues deactivated ACSS2 using two methods: CRISPR-Cas9 gene editing and VY-3-135, a powerful ACSS2 inhibitor discovered by Schug and his colleagues in 2021.

In this preclinical trial, the researchers discovered that targeting ACSS2 not only impeded this aggressive cancer’s capacity to metabolize acetate and grow, but it also stimulated the immune system to recognize and destroy the disease. Because cancer cells with suppressed ACSS2 cannot digest acetate properly, the tumor site becomes saturated in acetate, alerting the immune system to a problem.
Other TNBC experts have been perplexed by this “immunosensitization” approach of directing the immune system to the disease. However, Schug’s technique demonstrated that ACSS2 inhibition immunosensitized against TNBC so well that tumor development was substantially inhibited, even to the point of totally eliminating the cancer in several trials.
“Basically, we’ve proved that the immune system can take advantage of acetate that the tumor can’t process. It kicks the cancer while it’s down,” said Schug. “In fact, the immune system does this so well that it remembers how to attack TNBC in the future — even if that tumor’s ACSS2 gene is still active.”
Another group’s ACSS2-inhibiting technique is currently in human clinical trials, and Schug’s research illustrates how ACSS2-inhibiting treatment may be able to improve results for individuals with the dreaded TNBC. Schug et al. discovered that by evaluating ACSS2 inhibitors alongside regular anti-breast-cancer chemotherapy, ACSS2 inhibition improved treatment efficacy.
“We felt ACSS2 was a good target for TNBC. Our findings show how the immune effects of ACSS2 inhibition could be used in the treatment of TNBC patients with limited treatment options,” said Schug. “More research is needed, but we expect to see significant improvements in treating TNBC by combining this approach with other cancer therapies.”