Although there is a repair response associated with neurogenesis after a stroke, brain tissue is not regenerated. Failure to regenerate functional brain tissue is not due to a lack of available neural cells, but rather to a lack of structural support to allow repopulation of the lesion cavity. Researchers show in a zebrafish model that two proteins prevent scar formation in the brain, improving tissue regeneration.
LMU researchers show in a zebrafish model that two proteins prevent scar formation in the brain, improving tissue regeneration.
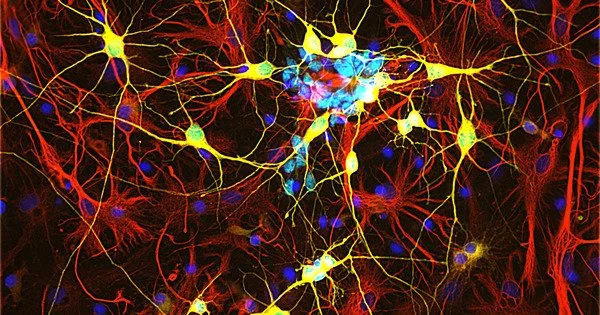
Whereas cells in most endogenous tissues renew themselves on a regular basis, the number of nerve cells in the human brain and spinal cord remains constant. Although adult mammalian brains can regenerate nerve cells, as LMU scientist Professor Magdalena Götz previously demonstrated, young neurons in brain injury patients are unable to integrate into existing neural networks and survive outside of two specific areas of the brain. This appears to be due to glial cells, which form the brain’s supporting tissue.
Microglia, in particular, cause inflammation and scarring, which isolate the injured site from the healthy brain but, in the long run, prevent proper incorporation of new neurons into the circuitry. It was previously unknown how the body regulates such mechanisms.
The goal was to tease out the differences between zebrafish and mammals in order to understand which signaling pathways in the human brain inhibit regeneration and how we might be able to intervene.
Professor Magdalena Götz
Now a team led by LMU cell biologist Prof. Jovica Ninkovic has demonstrated in Nature Neuroscience that reducing the reactivity of microglia is crucial to preventing chronic inflammations and tissue scars and thus to improving regeneration capability.
How CNS injuries heal in zebrafish
In contrast to mammals, the zebrafish’s central nervous system (CNS) has exceptional regenerative abilities. In the event of an injury, neural stem cells, among other things, generate long-lived neurons. Furthermore, CNS injuries cause only transient reactivity of glial cells in zebrafish, allowing nerve cells to integrate into injured tissue regions. “The goal was to tease out the differences between zebrafish and mammals in order to understand which signaling pathways in the human brain inhibit regeneration and how we might be able to intervene,” Ninkovic explains.
The scientists deliberately inflicted CNS lesions in zebrafish, prompting the activation of microglia. At the same time, the researchers found an accumulation of lipid droplets and TDP-43 condensates in the lesions. To date, the protein TDP-43 has been primarily associated with neurodegenerative diseases.
Granulin was also important in the zebrafish model. This protein helped to remove the lipid droplets and TDP-43 condensates, allowing the microglia to transition from an activated to resting state. The result was the regeneration of the injury without scarring. In contrast, zebrafish with experimentally induced granulin deficiency showed poor regeneration of the injury, similar to what we see in mammals. “We suspect that granulin plays an important role in nerve regeneration in zebrafish,” Ninkovic says.
From basic research to application
Ninkovic’s team investigated material from patients who died from brain injuries to further investigate the comparison between humans and zebrafish. There was also a link between the level of activation of microglia and the accumulation of lipid droplets and TDP-43 condensates. As a result, the corresponding signaling pathways in human tissue were comparable to those in zebrafish.
“Potential for novel therapeutic applications in humans,” according to the LMU researcher. He intends to investigate whether known low-molecular-weight compounds are suitable for inhibiting signaling pathways of microglia activation, thereby promoting neural lesion healing. In this pre-clinical phase, zebrafish models will be used once more.