Chemical stability is an important factor to consider when evaluating pharmacological characteristics, activity, and selectivity during drug discovery. Therapeutic development that leads to robust and marketable lead candidates remains a difficult scientific undertaking, as the move from a screening hit to a drug candidate necessitates knowledge and experience. For many years, natural products and their derivatives have been recognized as a source of medicinal agents and structural diversity.
Natural goods such as plants, animals, and minerals have long been used to heal human ailments. Nonetheless, ancient wisdom has served as the foundation for modern medicine and will continue to be an important source of future medicine and therapies.
What is the connection between gunpowder, penicillin, and Teflon? They were world-changing inventions, but they were all created entirely by chance. In a new study published in the journal Science, researchers utilized electricity to create a tool that could make it easier and less expensive to create the molecules used in medications and other natural products. Nonetheless, this invention joins the ranks of many unexpected innovations that have come before it.
We’re going to exploit this really reactive intermediate and see how far we can push it. It’s a very attractive way to do chemistry these days, because we have total control over how we run these reactions.
Christo Sevov
Christo Sevov, co-author of the work and an assistant professor of chemistry and biochemistry at The Ohio State University, was part of a team that first attempted to develop a catalyst that could be triggered by electricity to form the bonds of the targeted drug molecules.
The outcomes of their investigation reveal a general rule for employing inexpensive and readily available components to produce complicated combinations that would not ordinarily operate together. Streamlining this chemical process could allow researchers to safely manufacture more valuable goods with fewer steps and less waste.
Instead of employing high-energy reagents or adding compounds to enhance their chemical reactions in the lab, Sevov’s team used the power of electricity. Because electricity is environmentally sustainable, there has recently been a movement in the industrial sector to shift toward the use of electrochemistry to encourage chemical transformation.
“It’s a very attractive way to do chemistry these days, because we have total control over how we run these reactions,” Sevov said.
The research has broad applications in medicine, and in the creation of products like agrichemicals (like pesticides or herbicides) and certain plastics. But Sevov’s discovery, while seemingly serendipitous, took lots of hard work and patience to get right.

“It took maybe three months of testing different additive combinations before something worked and functioned incredibly well,” Sevov said. “Being able to get to that complex allowed us to put together materials that are really tough to stitch together under regular conditions.”
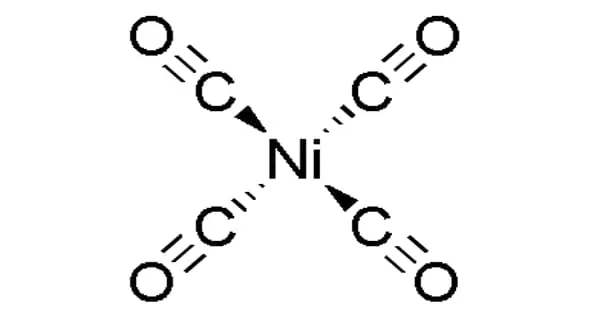
Because the precious metals commonly used as catalysts by chemists can be quite expensive, Sevov’s team chose a nickel atom as the catalyst for their tool. Catalysts in chemistry are responsible for boosting or reducing the rate of a chemical process by making and breaking bonds.
“The ability to employ relatively affordable catalysts, such as nickel, is tremendously useful to everyone in the entire community in general,” he said. Nickel is not just a low-cost alternative for companies that manufacture medications, plastics, and polymers; it also lowers the cost of food goods. If farmers had to pay more for the agrichemicals produced by these chemical processes, the price of their crop would climb accordingly, according to Sevov.
To expand on its findings, the team will work with Merck, a worldwide pharmaceutical company, to develop new medicines based on more challenging processes and complicated chemicals. But with their latest discovery, Sevov said that he’s optimistic that their work will start to create brand new avenues in the field of chemistry.
“We’re going to exploit this really reactive intermediate and see how far we can push it,” Sevov added.
Taylor Hamby and Matthew Lalama of Ohio State are among the co-authors. The National Institutes of Health provided funding for this study.