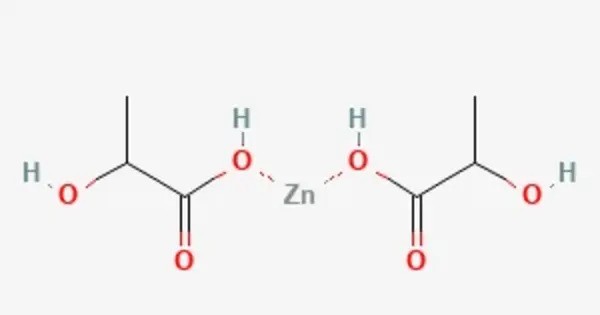
Zinc lactate is a chemical compound, a salt of zinc and lactic acid with the formula Zn(C3H5O3)2. It is a compound formed from the combination of zinc and lactic acid. It is commonly used as a source of zinc in dietary supplements, and it has a variety of applications in the food, cosmetic, and pharmaceutical industries.
Properties
Zinc lactate appears as a white to almost white fine powder. It is nearly odourless, highly soluble in water, and insoluble in ethanol. It forms dihydrates with the chemical formula Zn(C3H5O3)2 • 2H2O. It is water-soluble, which makes it easy to incorporate into liquid-based products or supplements.
- Chemical formula: C6H10ZnO6
- Molar mass: 245.5
- Appearance: White crystals
- Melting point: 277 °C (531 °F; 550 K)
- Solubility in water: Soluble
Synthesis
Reaction of lactic acid with zinc oxide:
2CH3CH(OH)COOH + ZnO → Zn(C3H5O3)2 + H2O
Use
- Dietary Supplements: Zinc lactate is used as a zinc source in various supplements. Zinc is an essential mineral that supports immune function, wound healing, DNA synthesis, and other vital bodily functions.
- Food Industry: It is sometimes used as a fortification ingredient in food products to enhance their nutritional value, particularly in products designed for individuals with zinc deficiencies.
- Cosmetic and Personal Care Products: Zinc lactate can be found in skin care products because of its soothing, anti-inflammatory properties, and its role in controlling acne by regulating oil production.
Health Benefits
- Immune Support: Zinc is crucial for immune function and may help reduce the duration of colds and infections.
- Skin Health: Zinc plays a role in collagen synthesis, which is important for wound healing and maintaining skin health.
- Cognitive Function: Zinc is involved in various cognitive processes, including memory and learning.
- Antioxidant Effects: Zinc acts as an antioxidant, protecting cells from oxidative damage.