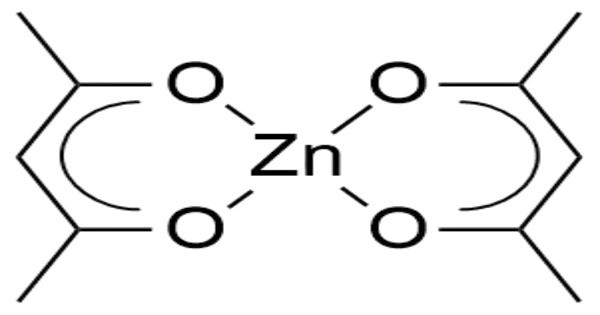
Zinc acetylacetonate is an acetylacetonate complex of zinc, with the chemical formula of Zn(C5H7O2)2. It consists of a zinc ion coordinated to two acetylacetonate ligands. The compound is in fact a trimer, Zn3(acac)6, in which each Zn ion is coordinated by five oxygen atoms in a distorted trigonal bipyramidal structure. This compound is often used as a catalyst in organic reactions and as a precursor in the synthesis of various zinc-containing materials.
It is usually a solid at room temperature and is soluble in organic solvents. It’s valued in applications such as polymerization processes, as a catalyst in reactions involving organometallic compounds, and in the preparation of thin films.
Preparation
Zinc acetylacetonate can be obtained by reacting zinc sulfate, acetylacetone and sodium hydroxide.
Properties
Zinc acetylacetonate is typically a white to yellowish solid. It is soluble in organic solvents like ethanol and acetone but less soluble in water. It is a crystalline substance that is slightly soluble in water.
- Chemical formula: C10H14O4Zn
- Molar mass: 263.60 g·mol−1
- Appearance: crystals
- Density: 1.41 g·cm−3
- Melting point: 124–126 °C
- Boiling point: 129–131 °C (13 hPa)
- Solubility in water: 6.9 g/L
- Solubility: soluble in organic solvants
Natural Occurrence
Zinc acetylacetonate is not typically found in nature as a standalone compound. However, zinc and acetylacetone (the ligand) are both naturally occurring substances.
Synthetic Production
It is primarily synthesized in laboratories or industrial settings. The synthesis often involves the reaction of zinc salts with acetylacetone.
Applications
- Catalyst: It is used as a catalyst in various organic reactions, including polymerization and esterification processes.
- Stabilizer: In some formulations, it serves as a stabilizer for plastics and rubber, helping to improve their thermal stability.
- Precursor: It’s also used in the synthesis of zinc oxide and other zinc-containing materials, which have applications in electronics, pigments, and pharmaceuticals.
Safety
Zinc acetylacetonate should be handled with care, as it may cause irritation to the skin and eyes. Proper safety protocols should be followed when working with this compound.