Researchers at Tokyo Tech have demonstrated that in-cell engineering can be a potent method for creating functional protein crystals with intriguing catalytic capabilities. The researchers created hybrid solid photosynthesis catalysts using genetically engineered bacteria as an eco-friendly synthesis platform. High activity, stability, and durability are displayed by these catalysts, demonstrating the potential of the suggested novel technique.
Protein crystals are well-ordered molecular structures with a variety of features and a great deal of room for customisation, just like ordinary crystals. They can assemble naturally from components found inside cells, which not only drastically lowers the cost of synthesis but also has a smaller negative influence on the environment.
Although the ability of protein crystals to house a variety of functional molecules makes them potential catalysts, existing methods can only bind tiny molecules and simple proteins.
To fully utilize protein crystals’ potential for enzyme immobilization, it is crucial to discover strategies to generate protein crystals containing both natural enzymes and synthesized functional molecules.
Against this backdrop, a team of researchers from Tokyo Institute of Technology (Tokyo Tech) led by Professor Takafumi Ueno has developed an innovative strategy to produce hybrid solid catalysts based on protein crystals.
As explained in their paper published in Nano Letters on 12 July 2023, their approach combines in-cell engineering and a simple in vitro process to produce catalysts for artificial photosynthesis.
The conversion efficiency of the proposed hybrid crystal was an order of magnitude higher than that of previously reported compounds for enzymatic artificial photosynthesis based on FDH. Moreover, the hybrid PhC remained in the solid protein assembly state after enduring both in vivo and in vitro engineering processes, demonstrating the remarkable crystallizing capacity and strong plasticity of PhCs as encapsulating scaffolds.
Professor Takafumi Ueno
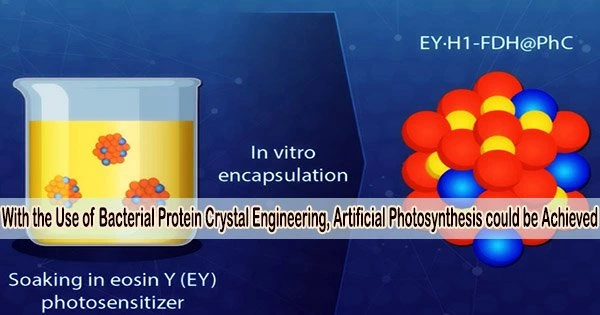
A protein monomer produced from a virus that infects the Bombyx mori silkworm serves as the foundation of the hybrid catalyst. The researchers introduced the gene that codes for this protein into Escherichia coli bacteria, where the produced monomers formed trimers that, in turn, spontaneously assembled into stable polyhedra crystals (PhCs) by binding to each other through their N-terminal α-helix (H1).
Additionally, the researchers introduced a modified version of the formate dehydrogenase (FDH) gene from a species of yeast into the E. coli genome. This gene caused the bacteria to produce FDH enzymes with H1 terminals, leading to the formation of hybrid H1-FDH@PhC crystals within the cells.
The scientists used sonication and gradient centrifugation to separate the hybrid crystals from the E. coli bacteria, and then they submerged them in a solution containing the synthetic photosensitizer eosin Y (EY).
As a result, the genetically altered protein monomers that could accommodate an eosin Y molecule in their central channel made it easier for large amounts of EY to bind to the hybrid crystal.
Through this ingenious process, the team managed to produce highly active, recyclable, and thermally stable EY·H1-FDH@PhC catalysts that can convert carbon dioxide (CO2) into formate (HCOO−) upon exposure to light, mimicking photosynthesis. In addition, compared to the free enzyme, they retained 94.4% of their catalytic activity following immobilization.
“The conversion efficiency of the proposed hybrid crystal was an order of magnitude higher than that of previously reported compounds for enzymatic artificial photosynthesis based on FDH,” highlights Prof. Ueno. “Moreover, the hybrid PhC remained in the solid protein assembly state after enduring both in vivo and in vitro engineering processes, demonstrating the remarkable crystallizing capacity and strong plasticity of PhCs as encapsulating scaffolds.”
Overall, this study demonstrates how bioengineering can make it easier to synthesize sophisticated functional materials.
“The combination of in vivo and in vitro techniques for the encapsulation of protein crystals will likely provide an effective and environmentally friendly strategy for research in the areas of nanomaterials and artificial photosynthesis,” concludes Prof. Ueno.