Antarctica, the southernmost continent, is known for its extreme cold and harsh conditions. Despite its inhospitable environment, various microorganisms, including bacteria, have adapted and managed to thrive there. Antarctic bacteria are fascinating subjects of study, offering insights into how life can persist in such extreme conditions.
For the first time, scientists have used extensive computer simulations to successfully forecast how to modify the optimal temperature of an enzyme. The starting point was an Antarctic bacterium’s cold-adapted enzyme.
Researchers from the Universities of Uppsala and Tromsø collaborated on the study, which was released in the journal Science Advances.
For example, ice-dwelling fish and bacteria both contain the kind of cold-adapted enzymes that the researchers used in their study. They have evolved to be able to work even at extremely low temperatures, where other enzymes would ordinarily be rendered inert.
Additionally, these enzymes always have a lower melting point and optimal temperature than enzymes from warm-blooded mammals and other creatures that can endure greater temperatures.
For example, this may involve creating new enzymes that catalyze chemical reactions not found in nature or changing their properties so that they can better cope with heat, cold, high pressure, increased salinity and so on. This area is therefore the subject of great biotechnological interest.
Professor Johan Åqvist
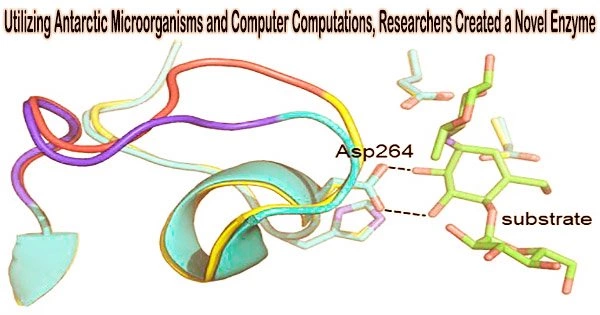
The scientists questioned whether computer models of the catalyzed chemical reaction could forecast a modest number of mutations in the Antarctic enzyme that may raise its optimal temperature. According to the calculation’s findings, this would be feasible if 16 mutations from the homologous pig enzyme were introduced into the bacterial form.
The scientists subsequently created this hybrid enzyme, tested its catalytic activity as a function of temperature, and discovered that it was faster than both the Antarctic and pig enzymes at 50°C and had an optimal temperature that was 6°C higher than the original variety.
Additionally, they used X-ray crystallography to solve the three-dimensional structure of the hybrid enzyme and demonstrate that the necessary structural alterations anticipated by the computer simulations had in fact occurred.
Researchers continue to study Antarctic bacteria to unravel the mysteries of their survival strategies and to understand the broader implications for ecology, biotechnology, and astrobiology.
In recent years, computer-based enzyme design has grown to be a significant and actively researched field. It is intended to develop enzymes with novel properties without the need for time-consuming tests or laborious computations.
“For example, this may involve creating new enzymes that catalyze chemical reactions not found in nature or changing their properties so that they can better cope with heat, cold, high pressure, increased salinity and so on. This area is therefore the subject of great biotechnological interest,” notes Johan Åqvist, Professor of Theoretical Chemistry at Uppsala University.