Polymer recycling can be defined as activities that can recover matter and/or energy from post-consumer plastics. Polymer recycling options range from mechanical recycling to chemical recycling and energy recovery. Mechanical recycling allows for the production of secondary polymers through mechanical processes, whereas chemical recycling allows monomers to be used to synthesize virgin polymers, monomer mixtures, or low molecular weight gases for energy recovery.
Polymers are lightweight, durable, and easily processed into fabricated parts, which helped polymers become the most relevant class of engineering materials by volume. Recycling polymers, on the other hand, is a problem that materials scientists have been working on for decades.
Increasing the service lifetime of polymers is an alternative path to a more sustainable polymer industry. The ability to “self-heal” from structural damage is an intriguing new idea. In collaboration with Krzysztof Matyjaszewski, professor of chemistry, Michael Bockstaller, professor of materials science and engineering at Carnegie Mellon University Materials Science and Engineering, discovered that the binding of copolymers on the surface of nanoparticles that are already used in industrial manufacturing provides an economical and scalable route toward self-healing polymers with increased strength and toughness.
If we can put polymers on the surface of nanoparticles, we can improve the interactions between them and make materials more mechanically robust and easier to form. This work illustrates how controlling macromolecular architecture can dramatically enhance properties of various advanced materials.
Michael Bockstaller
Atoms are typically thought of as the building blocks of materials. This concept inspired a new approach to fabricating functional materials in Bockstaller’s research group, which assembled nanoparticle building blocks using an atom transfer radical polymerization technique invented and developed by Matyjaszewski.
The properties of the resulting materials can be altered by manipulating the interactions between the nanoparticle building blocks. This concept opens up new possibilities for varying the properties of engineering materials without changing their chemical composition—a feature that is extremely beneficial in the context of recyclability.
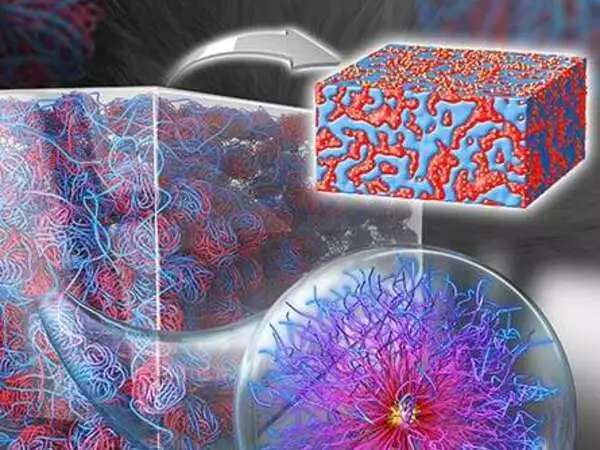
While working to make these particles more amenable to fabrication technologies like additive manufacturing, Bockstaller’s team experimented with putting copolymers at the surface of nanoparticles.
“If we can put polymers on the surface of nanoparticles, we can improve the interactions between them and make materials more mechanically robust and easier to form,” Bockstaller said. Matyjaszewski added, “This work illustrates how controlling macromolecular architecture can dramatically enhance properties of various advanced materials.”
Copolymers are a type of polymer that is made up of two different monomers and has self-healing properties. The researchers discovered that when copolymers were added to the surface of nanoparticles, new structures formed that improved the polymer’s self-healing properties. This discovery is critical for increasing polymer recyclability.
“This allows us to avoid material failure,” Bockstaller explained. “If the material can self-heal, we reduce the need to discard stress-damaged materials.” Bockstaller’s group will continue to investigate strategies for increasing the strength and toughness of copolymer-based self-healing materials and making them available for scalable production methods.