Mass spectrometry (MS) is an analytical technique used to determine the chemical composition of a sample by measuring the mass-to-charge ratio of charged particles. It is an analytical technique used to determine the mass-to-charge ratio of ions. The data are given as a mass spectrum, which is a plot of intensity vs mass-to-charge ratio.
Mass spectrometry is utilized in many different fields and can be applied to both pure samples and complex mixtures. This approach is frequently used in many scientific domains, including chemistry, biology, and physics. It gives significant information on a compound’s molecular weight, structure, and chemical characteristics.
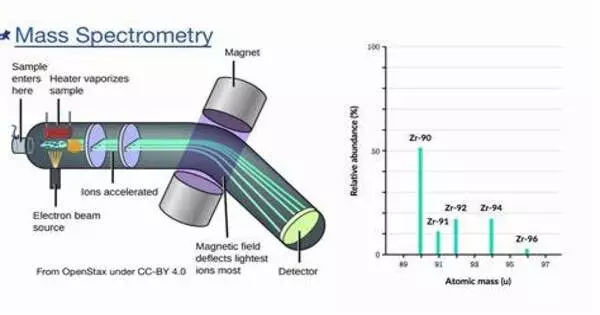
A sample, which might be solid, liquid, or gaseous, is ionized in a conventional MS method, for example, by bombarding it with an electron beam. Some of the molecules in the sample may break up into positively charged pieces as a result of this, or they may just become positively charged without fragmenting. These ions (fragments) are then sorted based on their mass-to-charge ratio, for example, by accelerating them and subjecting them to an electric or magnetic field: ions with the same mass-to-charge ratio will deflect in the same amount.
A mass spectrum is a plot of an ion signal as a function of mass-to-charge ratio. These spectra are used to determine a sample’s elemental or isotopic signature, particle and molecule masses, and the chemical identity or structure of molecules and other chemical compounds. A mass spectrometer’s fundamental components comprise a sample introduction system, an ionization source, a mass analyzer, and a detector. The process involves the following steps:
- Ionization: Ionization of the sample produces charged particles. Depending on the nature of the material, several ionization techniques such as electron ionization (EI), electrospray ionization (ESI), or matrix-assisted laser desorption/ionization (MALDI) can be utilized.
- Acceleration: The ions are accelerated into the mass analyzer, where they are separated according to their mass-to-charge ratio (m/z).
- Separation: The mass analyzer separates the ions depending on their mass-to-charge ratio, allowing individual ion detection.
- Detection: The detector captures each ion’s relative abundance as a mass spectrum, which is a depiction of the ion signal as a function of mass-to-charge ratio.
Applications
- Proteomics and Metabolomics: Identifying and quantifying proteins and metabolites in biological samples.
- Pharmaceutical Analysis: Determining the structure and quantity of pharmaceutical compounds in drug development and quality control.
- Environmental Analysis: Detecting pollutants and contaminants in air, water, and soil samples.
- Forensic Analysis: Analyzing trace evidence and identifying unknown substances in criminal investigations.