The development of medication resistance and subsequent disease progression is one of the most difficult concerns in cancer therapy. Moffitt Cancer Center researchers, in collaboration with Oxford University, report findings from a study using mathematical modeling to show that cell turnover influences drug resistance and is an important factor that governs the success of adaptive therapy in a new article featured on the cover of Cancer Research this month (16 February 2021).
Over the last few decades, cancer therapy choices have greatly expanded; unfortunately, many patients eventually acquire medication resistance. Physicians try to overcome resistance by either using a different technique to target cancer cells or by focusing on the resistance mechanism itself, but success with these treatments is generally limited since new resistance mechanisms emerge.
Resistance may emerge in part due to the large doses of medications frequently used during therapy, according to researchers from Moffitt’s Integrated Mathematical Oncology Department and Center of Excellence for Evolutionary Therapy. Typically, patients are given a maximum tolerated dose of therapy that kills as many cancer cells as feasible while causing the fewest side effects.
According to evolutionary theories, this maximum tolerated dose method could develop to drug resistance because drug-resistant cells exist before therapy begins. Drug-resistant cells are given free reign to divide and multiply after sensitive cells are eliminated by anti-cancer medicines. Adaptive therapy, an alternate therapeutic technique developed by Moffitt researchers, maybe a better way to kill cancer cells and prevent medication resistance, according to Moffitt researchers.
Adaptive therapy is an evolution-based therapeutic method that uses minimal effective drug doses or periodic drug holidays to preserve tumor volume. Finding the correct timing of treatment switch points is crucial for excellent adaptive therapy outcomes. Adaptive therapy dose and switch time points are made easier by mathematical models.
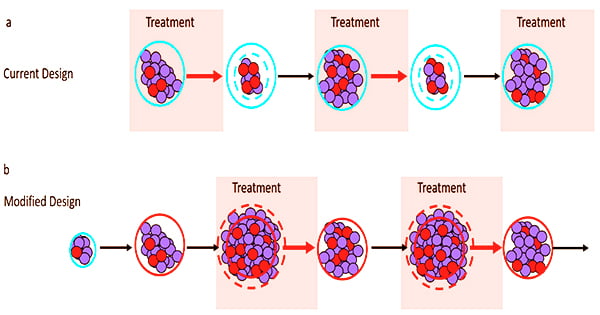
“Adaptive therapy aims not to eradicate the tumor, but to control it. Therapy is applied to reduce tumor burden to a tolerable level but is subsequently modulated or withdrawn to maintain a pool of drug-sensitive cancer cells,” said Alexander Anderson, Ph.D., chair of the Integrated Mathematical Oncology Department and founding director of the Center of Excellence for Evolutionary Therapy.
Adaptive therapy has been proven in the lab to postpone the time to cancer progression in a variety of tumor types, including ovarian, breast, and melanoma. In addition, clinical research conducted at Moffitt Cancer Center found that adaptive therapy prolonged the time to cancer progression by nearly 10 months and reduced cumulative drug usage by 53% when compared to normal treatment.
Despite these promising findings, it’s still unclear which tumor types may respond best to adaptive therapy in the clinic. Recent research has found that the success of adaptive therapy is influenced by a variety of parameters, including geographical limitation, the fitness of the resistant cell population, the initial number of resistant cells, and resistance mechanisms.
However, it’s unclear how the cost of resistance affects a tumor’s adaptive therapeutic response. When a medication is available, cells that develop resistance have a fitness advantage over non-resistant cells. However, this benefit may come at a cost, such as a decreased growth rate.
However, medication resistance is not always associated with a cost, and it is uncertain if a resistance cost is required for adaptive therapy to be successful. Moffitt’s study team employed mathematical modeling to figure out how the cost of resistance relates to adaptive therapy. They examined the time to disease progression in the presence and absence of a cost of resistance by modeling the formation of drug-sensitive and resistant cell populations under both continuous therapy and adaptive therapy scenarios.
Tumors with higher cell density and lower levels of pre-existing resistance performed better under adaptive therapy circumstances, according to the researchers. They also discovered that enhancing competition between sensitive and resistant cells increases cell turnover, which has an impact on the cost of resistance and adaptive therapy outcomes.
They did it by employing phase plane techniques, which allow them to visualize the dynamics of mathematical models.
“I’m a very visual person and find that phase planes make it easy to gain an intuition for a model. You don’t need to manipulate equations, which makes them great for communicating with experimental and clinical collaborators. We are honored that Cancer Research selected our collage of phase planes for their cover and hope this will help making mathematical oncology accessible to more people,” said Maximilian Strobl, lead study author and doctoral candidate at University of Oxford.
The researchers used data from 67 prostate cancer patients who were receiving intermittent therapy, a forerunner of adaptive therapy, to validate their models.
“We find that even though our model was constructed as a conceptual tool, it can recapitulate individual patient dynamics for a majority of patients and that it can describe patients who continuously respond, as well as those who eventually relapse,” said Anderson.
While additional research is needed to fully comprehend how adaptive therapies can benefit patients, experts are optimistic that their findings will lead to better markers of which malignancies will respond to adaptive therapy.
“With a better understanding of tumor growth, resistance costs, and turnover rates, adaptive therapy can be more carefully tailored to patients who stand to benefit from it the most and, more importantly, highlight which patients may benefit from multi-drug approaches,” said Anderson.