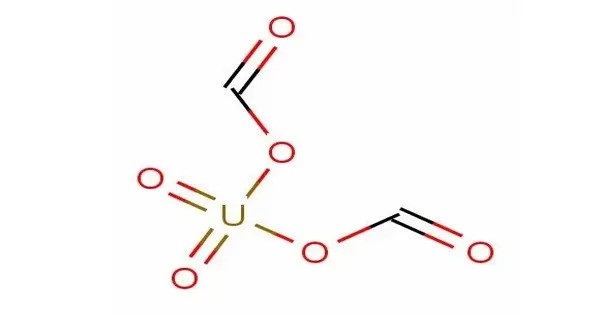
Uranyl formate (UO2(CHO2)2·H2O) is a salt that exists as a fine yellow free-flowing powder occasionally used in transmission electron microscopy. It typically forms a crystalline structure and is of interest in various fields, especially in nuclear chemistry and materials science, because of its uranium content and potential uses in radiation detection or as a precursor for further chemical synthesis.
It is occasionally used as a 0.5% or 1% aqueous negative stain in transmission electron microscopy (TEM) because it shows a finer grain structure than uranyl acetate. However, uranyl formate does not easily go into solution, and once dissolved, has a rather limited lifetime as a stain. It is quite sensitive to light, especially ultraviolet light, and will precipitate if exposed.
Properties
It is typically soluble in water, though the exact solubility depends on the specific conditions (temperature, concentration, etc.). The compound is relatively stable in dry conditions but can undergo hydrolysis in water, leading to the formation of more complex uranyl salts and species.
- Chemical formula: (UO2(CHO2)2·H2O)
- Molar mass: 378.08 g/mol
- Appearance: fine yellow powder
- Melting point: 110 °C (230 °F; 383 K)
Natural Occurrences
Uranyl formate is not typically found in large quantities in nature. However, compounds containing the uranyl ion are common in uranium ore deposits, and they may form as part of a suite of uranium minerals. The formate ion can be derived from natural organic material, potentially contributing to the formation of uranyl formate under certain conditions.
Laboratory Synthesis
Uranyl formate is often synthesized in laboratories, particularly in studies related to uranium chemistry, radioactive materials, and crystallography. It may be created by reacting uranium compounds like uranyl acetate or uranyl nitrate with formic acid or formate salts.
Uranium Processing
In the processing of uranium ores, uranyl formate can be an intermediate product. The compound may form during extraction or purification processes, especially those involving organic solvents or formic acid derivatives.
Environmental Relevance
The presence of uranium compounds, including uranyl formate, can have significance in environmental contexts, particularly in contaminated sites or areas affected by nuclear industry activities. Uranium compounds are studied in the context of groundwater contamination and radioactive waste management.