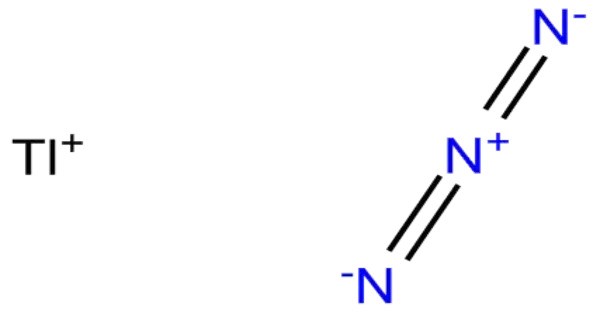
Thallium azide, TlN3, is a yellow-brown crystalline solid poorly soluble in water. Although it is not nearly as sensitive to shock or friction as lead azide, it can easily be detonated by a flame or spark. It can be stored safely dry in a closed non-metallic container. It is a toxic and potentially hazardous compound, and it has been used in certain applications, including as a source of thallium in chemical reactions, but it also poses serious health and environmental risks due to its toxicity.
Properties
- Chemical formula: TlN3
- Molar mass: 246.40 g·mol−1
- Appearance: yellow-brown crystals
- Solubility in water: insoluble
- Stability: It is highly unstable and can decompose violently, especially when heated or shocked. This is due to the instability of the azide group (N₃), which is known for its explosive properties.
Preparation and structure
Thallium azide can be prepared treating an aqueous solution of thallium(I) sulfate with sodium azide. Thallium azide will precipitate; the yield can be maximized by cooling.
TlN3, KN3, RbN3, and CsN3 adopt the same structures. The azide is bound to eight cations in an eclipsed orientation. The cations are bound to eight terminal N centers.
Occurrence
Thallium azide does not occur naturally in large amounts. Instead, it is synthesized in laboratories or industrial settings, often for specialized applications. It can be created by reacting thallium salts (such as thallium(I) nitrate) with sodium azide (NaN₃), a common precursor of azide compounds.
Thallium azide is sometimes used in the manufacture of detonators or other explosive devices because of its sensitivity to shock or heat.
Hazards
- Health Hazards: Thallium azide is highly toxic and can be fatal if ingested, inhaled, or absorbed through the skin. It can cause severe damage to the liver, kidneys, and nervous system.
- Environmental Impact: Due to its toxicity, thallium azide is considered hazardous to the environment, particularly to aquatic life.
Uses
Thallium azide has been used in specialized chemical processes but is mostly avoided in practical applications due to the risks associated with handling such toxic materials.
Safety
All thallium compounds are poisonous and should be handled with care. Azide salts are also roughly as toxic as their corresponding cyanide salts.