Tannic Acid
Definition
Tannic acid is a lustrous, yellow-brown, amorphous tannin, having the chemical composition C76H52O46. Commercial tannic acid is usually extracted from any of the following plant parts: Tara pods (Caesalpinia spinosa), gallnuts from Rhus semialata or Quercus infectoria or Sicilian Sumac leaves (Rhus coriaria). Tannins are polyphenols, and form yellowish to light brown amorphous masses that can be powdery, flaky, or spongy. They are used in photography, dyeing, in tanning leather, in clarifying wine and beer, and as an astringent in medicine. Tannins are also an important ingredient in tea.

Tannins occur normally in the roots, wood, bark, leaves, and fruit of many plants, particularly in the bark of oak species and in sumac and myrobalan. They also occur in galls, pathological growths resulting from insect attacks.
Tannic acid is not an appropriate standard for any type of tannin analysis because of its poorly defined composition. It is a polyphenolic compounds with molecular weights of around 500-3000 daltons and containing enough hydroxyl groups (1-2 per 100 MW) for effective cross linking of other compounds (ASTRINGENTS).
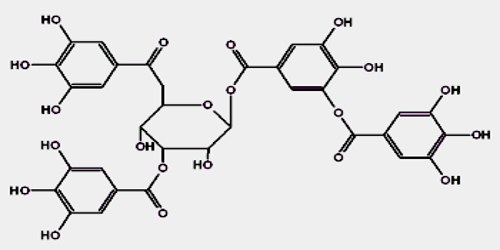
Tannins may be classified chemically into two main groups, hydrolyzable and condensed. Hydrolyzable tannins decomposable in water, with which they react to form other substances, yield various water-soluble products, such as gallic acid and protocatechuic acid and sugars. Gallotannin, or common tannic acid, is the best known of the hydrolyzable tannins. Condensed tannins, the larger group, form insoluble precipitates called tanner’s reds, or phlobaphenes. Among the important condensed tannins are the extracts from the wood or bark of quebracho, mangrove, and wattle.
Applications of Tannic Acid
Tannic acid is one type of tannin that has a fairly weak acidity. This substance is usually found as a yellow, white, or light brown powder that tends to easily dissolve in water. It is also used for industrial purposes, such as leather production and wood staining.

In addition to their principal applications in leather manufacture and dyeing, tannins are used in the clarification of wine and beer, as a constituent to reduce viscosity of drilling mud for oil wells, and in boiler water to prevent scale formation. Because of its styptic and astringent properties, tannin has been used to treat tonsillitis, pharyngitis, hemorrhoids, and skin eruptions; it has been administered internally to check diarrhea and intestinal bleeding and as an antidote for metallic, alkaloidal, and glycosidic poisons, with which it forms insoluble precipitates. Soluble in water, tannins form dark blue or dark green solutions with iron salts, a property utilized in the manufacture of ink.
Similarly tannic acid can also be used as an aftertreatment to improve wash fastness properties of acid dyed polyamide. It is also an alternative for fluorcarbon aftertreatments to impart anti-staining properties to polyamide yarn or carpets. Tannic acid is also found in commercially available iron/steel corrosion treatments, such as Hammerite Kurust.

One disadvantage is that it is believed to interfere with iron absorption. Iron is a mineral essential to maintaining proper human health, particularly with building strong red blood cells. Tannic acide may also have a negative effect on the digestive process. Use of this substance has been associated with severe liver and kidney damage.
This substance is also commonly used to tan animal skin to produce leather, and it is often stated that tanning was the first use that humans made of it.
Reference: