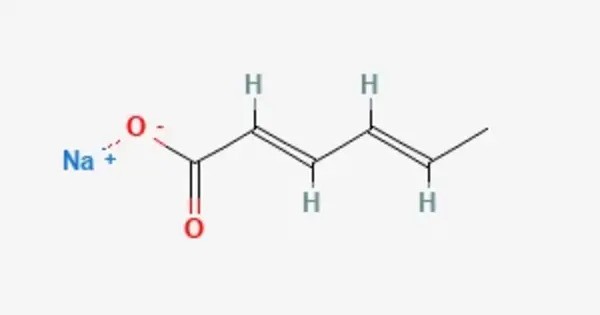
Sodium sorbate is the sodium salt of sorbic acid. It is an unstable white solid. It’s commonly used in the food, beverage, and cosmetic industries to prevent the growth of mold, yeast, and some bacteria, extending the shelf life of products. Unlike other sorbic acid salts such as potassium sorbate (E202) and calcium sorbate (E203), the use of sodium sorbate as a food additive is prohibited in the EU due to potentially genotoxic effects. Its E-number is E201.
Sodium sorbate works by interfering with the metabolism of microbes, preventing them from multiplying. It is particularly effective against mold and yeast, though its antimicrobial activity is less pronounced against bacteria compared to other preservatives like sodium benzoate.
Properties
- Chemical formula: C6H7NaO2
- Molar mass: 134.10835 g/mol
- Odor: hydrocarbon-like
- Boiling point: 233 °C (451 °F; 506 K)
- Appearance: White, crystalline powder or granules. It is typically odorless or has a very mild, slightly astringent odor.
- Solubility: It is highly soluble in water and ethanol, making it useful in liquid form for food and cosmetic applications.
- pH: It is more stable in mildly acidic environments, which is why it is most often used in acidic products like fruit juices, pickles, and wines.
Effectiveness as a Preservative
Sodium sorbate works as an antimicrobial agent by disrupting the cell membranes of microorganisms, preventing their growth and reproduction. Its activity is generally effective in acidic environments (pH between 4-6), and it is less effective at higher pH.
Occurrence
Sodium sorbate does not naturally occur in large quantities but is synthetically produced from sorbic acid. Sorbic acid itself can be obtained from the berries of the Sorbus aucuparia (mountain ash) or Hippophae rhamnoides (sea buckthorn), where it occurs in small amounts.
- Synthetically Produced – Most sodium sorbate used commercially is synthesized through a chemical process that involves neutralizing sorbic acid with sodium hydroxide (NaOH). This synthetic route allows for large-scale production to meet the demand in the food and cosmetics industries.
- Naturally Occurring Precursors – As mentioned, sorbic acid can be derived from certain berries and plants, particularly the mountain ash (Sorbus aucuparia). However, this natural source is not significant for industrial production due to the relatively low concentrations of sorbic acid found in these plants.
Applications
- Food Industry: Sodium sorbate is widely used to preserve foods such as cheese, dried fruits, beverages, and baked goods. It prevents mold and yeast growth in these products.
- Cosmetics & Personal Care Products: It is used as a preservative in cosmetics, shampoos, lotions, and creams to prevent microbial contamination and extend shelf life.
- Pharmaceuticals: Sodium sorbate is sometimes used in pharmaceutical formulations to prevent microbial contamination.
- Wine and Beverage Industry: In the production of wines and fruit juices, sodium sorbate is used to prevent fermentation after bottling.
Toxicity
Sodium sorbate is considered safe for consumption and use in cosmetics and personal care products at concentrations up to 0.1%. It is classified as a GRAS (Generally Recognized As Safe) substance by the FDA when used in food. However, some people may experience skin irritation or allergic reactions.