In oil, sodium hydride is a silvery to whitish powder or slurry. It is the chemical compound with the empirical formula NaH. This alkali metal hydride is largely utilized in organic synthesis as a powerful but flammable base. In contrast to molecular hydrides such as borane, methane, ammonia, and water, NaH is a saline (salt-like) hydride made up of Na+ and H− ions. Ionic crystals, salt compositions in which the hydrogen is negative monovalent ions, include sodium hydride.
When heated, NaH decomposes without melting, and sodium hydride is hydrolyzed with water to produce sodium hydroxide and hydrogen. It’s an ionic substance that’s insoluble in organic solvents (yet soluble in molten Na), indicating that there are no H- ions in solution. Because of NaH’s insolubility, all reactions involving it take place at the solid’s surface. Pure sodium hydride is silver needle-like crystals, but commercially available sodium hydride commerce is usually a mild gray crystalline powder with a 25 percent to 50 percent sodium hydride concentration dispersed in oil. The relative density is 0.92.
The direct interaction of hydrogen and liquid sodium produces NaH. Although pure NaH is colorless, samples usually seem grey. NaH (0.968 g/cm3) is about 40% denser than Na. Sodium hydride has a crystalline rock salt structure (lattice constant a = 0.488nm), and hydrogen ion exists in anion form as lithium hydride in ionic crystalline. It decomposes explosively in water; reacts violently with lower alcohols; dissolves in molten sodium and molten sodium hydroxide; insoluble in liquid ammonia, benzene, carbon tetrachloride, and carbon disulfide; has a heat of production of 69.5kJ mol-1; and decomposes into metallic sodium and hydrogen at a high temperature of 800 °C.
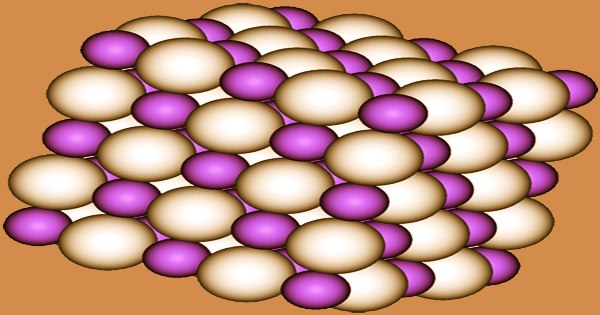
The NaCl crystal structure is adopted by NaH, LiH, KH, RbH, and CsH. Each Na+ ion is surrounded by six H− centers in an octahedral shape in this motif. According to the Na−H and Na−F distances, the ionic radii of H− (146 pm in NaH) and F− (133 pm) are comparable. Sodium hydride is a powerful reducing agent. For example, at 400 ℃, titanium tetrachloride can be reduced to metallic titanium:
TiCl4 = 4NaH + Ti + 4NaCl + 2H2.
It decomposes into hydrogen gas when heated to 425 °C at air pressure. It can also react aggressively with water, causing a fire and producing sodium hydroxide and hydrogen. It produces amine salt (sodium amide) and hydrogen when it combines with liquid ammonia. In a molecule known as “inverse sodium hydride,” which contains both H+ and Na−ions, an unexpected scenario develops. Na− is an alkalide with a substantially larger energy content than conventional sodium hydride due to the net displacement of two electrons from hydrogen to sodium.
Sodium hydride reacts with halogen, sulfur vapor, sulfur dioxide, and carbon dioxide at high temperatures. It has a strong reductive capacity and can liberate metal from metal oxides and metal chlorides. Theoretical work has suggested that under certain solvent circumstances, even an unprotected protonated tertiary amine complexed with sodium alkalide might be metastable, however the barrier to reaction would be tiny and finding a suitable solvent would be challenging. In organic chemistry, NaH is a versatile and useful basic. It can deprotonate a variety of even mild Brnsted acids to produce sodium derivatives when used as a superbase.
Sodium hydride is stable in dry air below 230°C, but it will burn into sodium oxide above this temperature. Even at low temperatures, if trace levels of sodium are present, it is easy to ignite. Water and organic fire extinguishing agents must not be utilized when shooting. Carbon acids (i.e., C-H bonds) such as 1,3-dicarbonyls, such as malonic esters, are deprotonated by NaH. The sodium derivatives that result can be alkylated. NaH is commonly used to induce the Dieckmann condensation, Stobbe condensation, Darzens condensation, and Claisen condensation reactions of carbonyl compounds.
Hydrogen gas is injected into molten sodium metal distributed in oil to produce sodium hydride. Alternatively, hydrogen can be injected into sodium that has been scattered throughout the surface of an inert material. Although NaH reduces certain main group compounds, similar reactivity in organic chemistry is uncommon. Boron trifluoride, for example, interacts to form diborane and sodium fluoride:
6 NaH + 2 BF3 → B2H6 + 6 NaF
In disilanes and disulfides, the Si-Si and S-S bonds are likewise decreased. The reaction of sodium with hydrogen at roughly 360 °C (680 °F) produces sodium hydride, which is reactive with water and produces hydrogen gas and NaOH solution. Used as a powerful reducing agent. A composite reagent comprised of sodium hydride and an alkali metal iodide (NaH:MI, M = Li, Na) can be used to perform a number of reduction processes, including the hydrodecyanation of tertiary nitriles, the reduction of imines to amines, and the reduction of amides to aldehydes.
Sodium hydride can be utilized as a condensation and alkylation catalyst, as well as a polymerization catalyst, in the pharmaceutical and fragrance industries. It may also be used to make boron hydrides, as well as reducing agents, condensing agents, desiccant, and Clay Johnson’s reagents. NaH is usually suspended in THF, a solvent that is resistant to strong bases yet can solvate a wide range of reactive sodium compounds. It can be used as a condensing agent, an alkylating agent, or a reducing agent, among other things. It’s a common reductant in pharmaceuticals, fragrances, and dyes, as well as a drying agent, an alkylating agent, and other applications.
Information Sources: