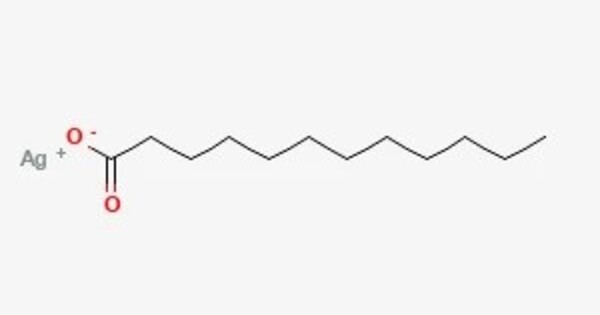
Silver laurate is an inorganic compound, a salt of silver and lauric acid with the formula AgC11H23COO, colorless (white) crystals. It is a compound formed by the combination of silver and lauric acid. Lauric acid is a saturated fatty acid found in coconut oil and palm kernel oil, and silver laurate is typically used in various applications, including as a lubricant or a component in some chemical formulations. It can also be used in certain specialty products, such as in the development of antimicrobial agents due to silver’s well-known antibacterial properties.
Silver laurate is a salt and can behave similarly to other metal carboxylates. It can react with acids or bases to release lauric acid and silver salts.
Properties
Silver laurate is typically a white or off-white solid. It is generally insoluble in water but soluble in organic solvents like ethanol and acetone. It is relatively stable under normal conditions but can decompose upon heating or exposure to light. It does not dissolve in ethanol or in diethyl ether.
- Chemical formula: C11H23AgO2
- Molar mass: 295.171 g·mol−1
- Appearance: Colorless (white) crystals
- Density: 1.5 g/cm3
- Melting point: 215.5 °C (419.9 °F; 488.6 K)
- Solubility in water: Insoluble
Synthetic Preparation
Silver laurate is typically synthesized in a laboratory setting. It is prepared by reacting lauric acid with silver nitrate in a controlled environment.
Applications
It’s used in various applications, including in the production of antimicrobial coatings and in some types of specialized catalysts. Its silver content makes it useful in contexts where antibacterial properties are desirable.
Natural Occurrence
Silver laurate does not naturally occur in significant quantities. It is mainly produced for specific industrial or research purposes rather than being found in nature.