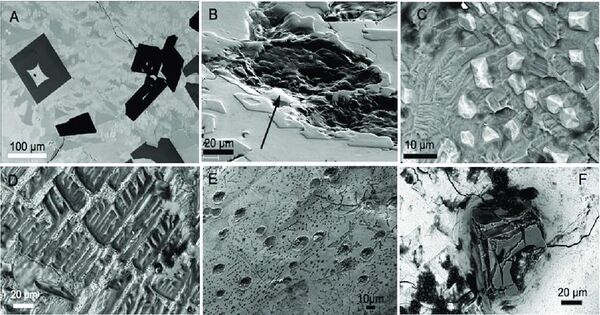
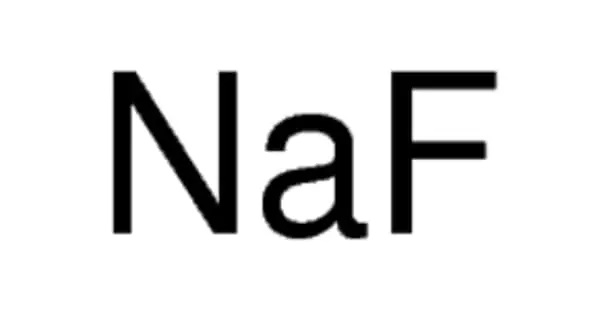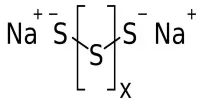Silicide carbides, also known as carbide silicides, are compounds that contain anions made up of silicide (Si4−) and carbide (C4−) or clusters thereof. This is a class of compounds that contain both silicide and carbide components. They can be classified as mixed anion compounds or intermetallic compounds, with silicon being a semimetal. They are primarily produced from silicon (Si) and carbon (C), and they have distinct features that make them helpful in a variety of applications, including materials science and engineering.
Related compounds include germanide carbides, phosphide silicides, boron carbides, and nitride carbides. Other similar compounds, such as carbidonitridosilicates with C(SiN3)4, may have more condensed anion pairings, with N bridging two silicon atoms.
Silicide carbides have the general formula SixCy, with x and y varying depending on the combination. They are usually made up of silicon and carbon, although they can sometimes contain additional elements such as transition metals.
Production
Silicide carbide compounds can be formed by melting silicon, graphite, and metal together. It is critical to exclude oxygen before and throughout the reaction. The flux method employs a reaction in molten metal. Gallium is appropriate since it dissolves carbon and silicon while not reacting with them.
Properties
Silicide carbides are ceramic materials with metallic characteristics. They are less brittle than most ceramics, yet more rigid than metals. They have a high melting temperature. Water has no effect on silicide carbide compounds in the air. The look is often metallic grey.
When powdered the colour is dark grey. When ErFe2SiC is dissolved in acid, mostly methane is produced, but the products include some hydrocarbons with two and three carbon atoms. The lanthanide contraction is evident with the cell sizes for rare earth element silicide carbides.
Applications
- Ceramics and Refractories: Silicide carbides are used in the production of advanced ceramics and refractory materials due to their high melting points and hardness.
- Coatings: They are applied as coatings on metals and other materials to enhance their wear resistance and thermal properties.
- Semiconductors: Certain silicide carbides have semiconductor properties, making them useful in electronics and optoelectronics.
Examples
- Tungsten Silicide Carbide (WSiC): Known for its excellent mechanical properties and high-temperature stability.
- Titanium Silicon Carbide (Ti3SiC2): A ternary layered ceramic material with good oxidation resistance and electrical conductivity.
- Molybdenum Silicide Carbide (MoSiC): Used in high-temperature structural applications due to its high melting point and strength.