EPFL researchers discovered that super-selective binding interactions between nanomaterials and protein surfaces are controlled not only by molecular density but also by pattern and structural rigidity. The discovery could help improve current approaches to virus prevention and cancer detection.
So much of biology is based on the biophysical process of binding: establishing a strong bond between one or more groups of atoms known as ligands and their corresponding receptor molecule on a surface. The first fundamental process that allows a virus to infect a host, or chemotherapy to fight cancer, is a binding event. But binding interactions—at least, our understanding of them—have a “Goldilocks problem”: too few ligands on one molecule makes it impossible for it to stably bind with the correct target, while too many can result in undesirable side effects.
“When binding is triggered by a threshold density of target receptors, we call this ‘super-selective’ binding, which is critical to preventing random interactions that could dysregulate biological function,” explains Maartje Bastings, head of the Programmable Biomaterials Laboratory (PBL) in the School of Engineering.
Our goal was to carve out design principles as simply as possible, so that every ligand molecule participates in the binding interaction. We now have a very useful toolbox for further exploiting super-selective binding interactions in biological systems.
Maartje Bastings
“Because nature does not overcomplicate things, we wanted to know the bare minimum of binding interactions that would still allow for super-selective binding to occur. We also wanted to know if the pattern in which the ligand molecules are arranged affects selectivity. As it turns out, it does.”
Bastings and four of her Ph.D. students have recently published a study in the Journal of the American Chemical Society that identifies the optimal ligand number for super-selective binding: six. But they also found, to their excitement, that the arrangement of these ligands—in a line, circle, or triangle, for example—also significantly impacted binding efficacy. They have dubbed the phenomenon “multivalent pattern recognition” or MPR.
“MPR opens up a whole new set of hypotheses around how molecular communication in biological and immunological processes might work. For example, the SARS-CoV-2 virus has a pattern of spike proteins that it uses to bind to cell surfaces, and these patterns could be really critical when it comes to selectivity.”
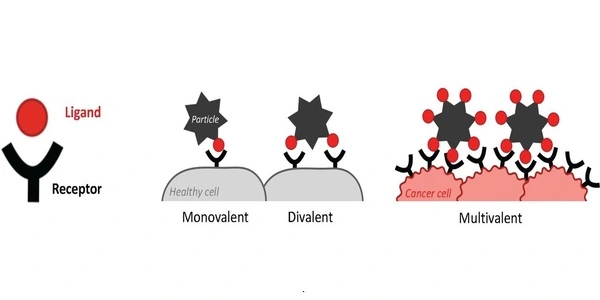
From coronaviruses to cancer
Because its double helix structure is so precise and well understood, DNA is the perfect model molecule for the PBL’s research. For this study, the team designed a rigid disk made entirely out of DNA, where the position and number of all ligand molecules could be precisely controlled. After engineering a series of ligand-receptor architectures to explore how density, geometry, and nano-spacing influenced binding super-selectivity, the team realized that rigidity was a key factor. “The more flexible, the less precise,” Bastings says.
“Our goal was to carve out design principles as simple as possible so that every ligand molecule participates in the binding interaction. We now have a very useful toolbox for further exploiting super-selective binding interactions in biological systems.”
The applications for such a “toolbox” are numerous, but Bastings sees three that are immediately useful. “Whether you like it or not,” she says, “the SARS-CoV-2 virus is currently the first thought when it comes to virological applications.” With our findings, one could imagine creating a super-selective particle with ligand patterns designed to bind with the virus to prevent infection or to block a cell site so that the virus cannot infect it.”
Super-selectivity may also benefit diagnostics and therapeutics such as chemotherapy, allowing for more reliable binding with cancer cells, which are known to have a higher density of certain receptor molecules. In this case, healthy cells would go undetected, significantly reducing side effects.
Finally, such selectivity engineering could provide important insights into complex interactions within the immune system. “Because we can now play precisely with patterns of what happens at binding sites, we can potentially ‘communicate’ with the immune system,” Bastings says.